SCREENING AND CONFIRMATION METHOD FOR VETERINARY DRUGS AND ADDITIVES IN ANIMAL-DERIVED FOOD
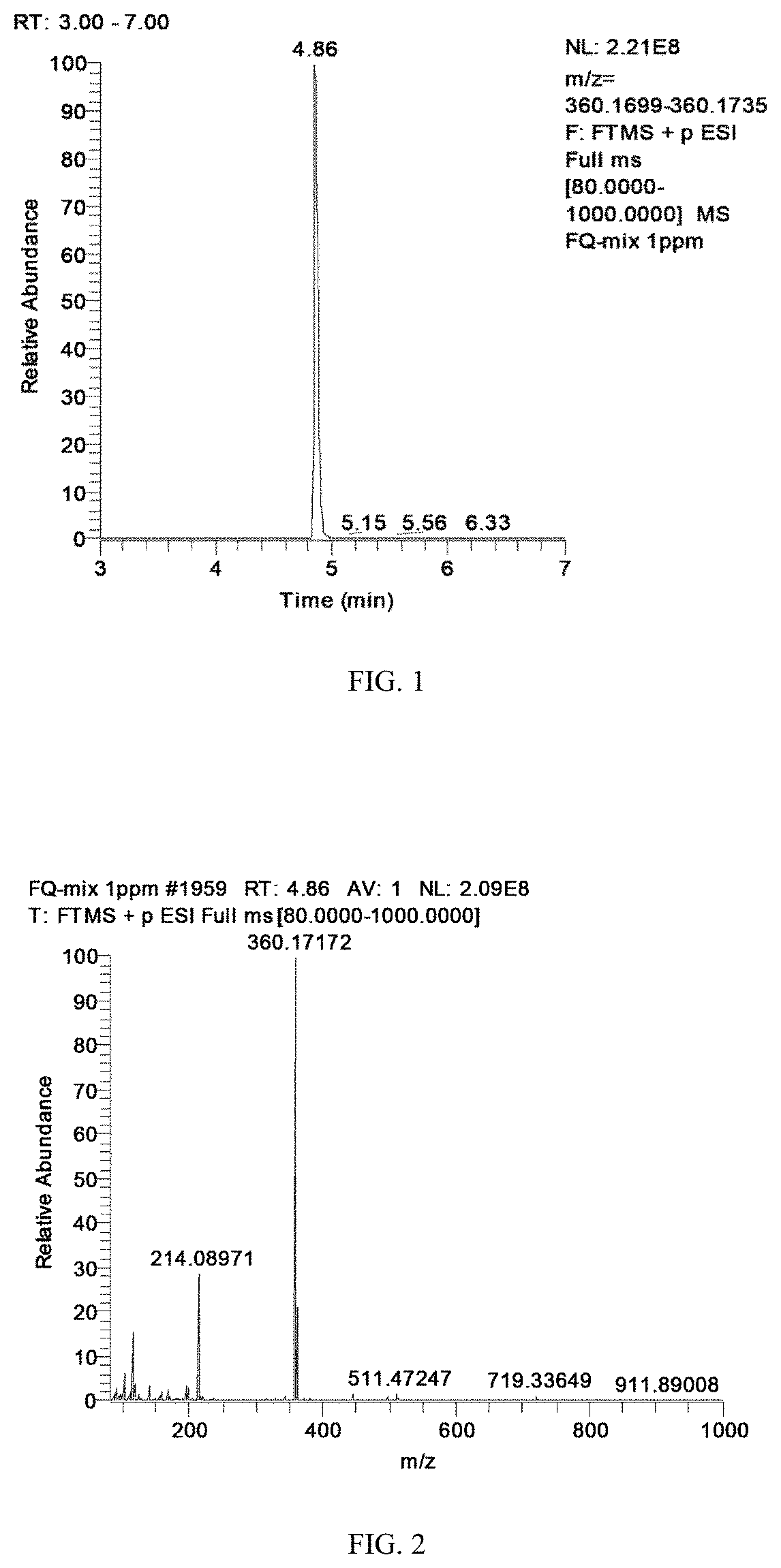
This application claims the benefit of priority from Chinese Patent Application No. 201910891387.7, filed on Sep. 20, 2019. The content of the aforementioned application, including any intervening amendments thereto, is incorporated herein by reference in its entirety. This application relates to detection of animal-derived food, and more particularly to a screening and confirmation method for veterinary drugs and additives in animal-derived food. Veterinary drugs refer to substances used to prevent, treat, diagnose animal diseases or purposely regulate animal physiological functions, which generally include antibiotics, anthelmintics, growth promoters, antiprotozoals, trypanosome-killing substances, sedatives and β-adrenoceptor blockers. Recently, the animal husbandry has achieved rapid development in China, which is attributed to the development and rational use of veterinary drugs. Veterinary drugs have played an important role in reducing the morbidity and mortality of livestock, promoting animal growth, improving the quality of animal-derived products and improving feed utilization. However, at present, the veterinary drugs are often abused with the dosage far exceeding the therapeutic dose for animal diseases, resulting in drug residues in the animal-derived food. Through the long-term accumulation, most of the veterinary drugs show significant toxicity. For example, residual lincosamines can cause renal dysfunction and increased drug resistance of Gram-positive bacteria; residual macrolide drugs can cause allergic reactions and also lead to the spread of strains carrying drug-resistant factors; and chloramphenicol can cause anemia, gray baby syndrome and leukemia, etc. Therefore, the European Union and China have established markers for related drug residues and maximum residue limits in different animal-derived foods, and also stipulated the types of veterinary drugs banned from animal husbandry, but the abuse of veterinary drugs still cannot be precluded. Public notice No. 235 of the Ministry of Agriculture of China has clearly stipulated the maximum residue limit of each drug in animal tissues. However, the irrational and illegal use of veterinary drugs still can be found in the veterinary drug inspection or in the farms. In addition to veterinary drugs, the pesticide residues in animal foods also raised widespread concern in 2017 due to the “fipronil” poison egg incident. Since the residues of pesticides and veterinary drugs in animal-derived foods directly or indirectly endanger the human health, and destroy the ecological environment, it is necessary to comprehensively regulate and monitor the use of veterinary drugs and additives in animal-derived foods. The development of the detection technology enables the simultaneous detection of multiple categories of pesticides and veterinary drugs, and also diversifies the pre-processing technologies and instrument analysis tools. Developed countries such as Europe and the United States have made a great progress in the detection of residual pesticides and veterinary drugs. Specifically, the Food and Drug Administration (FDA) have established a method for detecting more than 150 kinds of veterinary drugs in milk using LC-TOF-MS, which has a detection limit of 10 ng/mL or lower for 50% of the veterinary drugs. The U.S. Department of Agriculture have established a detection method for 120 kinds of veterinary drugs in 11 categories in bovine kidney using HPLC-Quadrupole Mass Spectrometry, which has a detection limit of 1-100 ng/g and a recovery rate of 29%-192%. At present, the multi-residue detection in China is mainly performed by HPLC-Quadrupole Mass Spectrometry and liquid chromatography-high resolution mass spectrometry (LC-HRMS). Among them, the traditional HPLC-Quadrupole Mass Spectrometry has the characteristics of high sensitivity and accurate quantification. However, it also has the shortcomings of low resolution, high occurrence rate of false positives, limited detectable drugs and large complexity in the establishment of methods. An object of this application is to provide a screening and confirmation method for 207 veterinary drugs and additives in animal-derived food to solve the above problems. The technical solutions of the application are specifically described as follows. This application provides a screening and confirmation method for veterinary drugs and additives in animal-derived food, comprising: (1) dissolving 10 mg of each reference standard of the veterinary drugs and additives followed by diluting to 10 mL in a volumetric flask to prepare a 1 mg/mL stock solution of each reference standard; wherein the veterinary drugs and additives are selected from: (a) sulfonamides: sulfadimidine, sulformethoxine, sulfisoxazole, sulfaguanidine, sulfamerazine, sulfamethylthiadizaole, sulfamethoxazole, sulfamethoxydiazine, sulfamethoxypyridazine, sulfamonomethoxine, sulfamethazole, sulfapyridine, sulfaquinoxaline, sulfathiazole, sulfisomidine, trimethoprim, diaveridine, phthalylsulfathiazole, sulfabenzamide, sulfacetamide, sulfachlorpyridazine, sulfachloropyrazine, sulfadiazine and sulfadimethoxine; (b) fluoroquinolones: lomefloxacin, marbofloxacin, nadifloxacin, nalidixic acid, norfloxacin, ofloxacin, orbifloxacin, oxolinic acid, pipemidic acid, pefloxacin, cinoxacin, sarafloxacin, sparfloxacin, ciprofloxacin, danofloxacin, difloxacin, enoxacin, enrofloxacin, fleroxacin, flumequine and gatifloxacin; (c) benzimidazoles: mebendazole, mebendazole-amine, 2-aminofluorobenzimidazole, oxfendazole, oxibendazole, parbendazole, 5-hydroxythiabendazole, thiabendazole, albendazole, albendazole sulfone, albendazole sulfoxide, albendazole aminosulfone, benzimidazole, febantel and lobendazole; (d) stimulants: cyproheptadine, fenoterol, labetalol, penbutolol, phenylethanolamine A, ractopamine, salbutamol, salmeterol, terbutaline, mabuterol, tulobuterol, bambuterol, brombuterol, cimaterol, cimbuterol, clenbuterol, clenproperol and clonidine; (e) hormones: chlormadinone acetate, 17a-methyltestosterone, clobetasol propionate, clobetasone butyrate, corticosterone, hydrocortisone, cortisone acetate, cortisone, deflazacort, dexamethasone, diflorasone diacetate, epitestosterone, estradiol benzoate, fludrocortisone acetate, flumetasone pivalate, flumethasone, fluocinolone acetonide, fluorometholone, flurandrenolide, fluticasone propionate, nandrolone propionate, halcinonide, dihydrodiethylstilbestrol, medroxyprogesterone acetate, megestrol acetate, melengestrol acetate, methandienone, methylprednisolone, norethindrone, nandrolone propionate, levonorgestrel, prednicarbate, prednisolone, prednisone, progesterone, dehydrotestosterone, testosterone, trenbolone, triamcinolone acetonide, beclomethasone, betamethasone dipropionate, betamethasone valerate and betamethasone; (f) nitroimidazoles: dimetridazole, ipronidazole, metronidazole, ornidazole, hydroxydimetridazole, ronidazole, secnidazole and tinidazole; (g) antiviral substances: amantadine and rimantadine; (h) lincosamides: clindamycin and lincomycin; (i) amphenicols: chloramphenicol, florfenicol and thiamphenicol; (j) penicillins: ceftiofur, flucloxacillin, cloxacillin, nafcillin, piperacillin and penicillin G; (k) quinoxalines: quinocetone; (l) antipyretics and analgesics: phenacetin, salicylic acid, amidopyrine, antipyrine, dapsone, flunixin meglumine and acetaminophen; (m) psychoactive drugs: haloperidol, imipramine, nitrazepam, oxazepam, pemoline, perphenazine, promethazine, propionylpromazine hydrochloride, sulpiride, xylazine, acepromazine, anisopirol, azaperone, carbamazepine, chloropromazine, diazepam, diphenhydramine, droperidol, estazolam, clarithromycin, tiamulin, erythromycin, kitasamycin, tilmicosin and tylosin; (n) anthelmintics: toltrazuril sulfoxide, clopidol, dinitolmide, halofuginone and levamisole; (o) pesticides: buprofezin, carboxin, 3-hydroxycarbofuran, clothianidin, cyromazine, diuron, ethopabate, fipronil, fluridone, acephate, hexazinone, imazalil, imidacloprid, linuron, metribuzin, myclobutanil, acetamiprid, norflurazon, propyzamide, atrazine, simazine, desethyl atrazine, azoxystrobin and benoxacor; (p) other substances: scopolamine, valnemulin and atropine; the norfloxacin is dissolved with water and diluted with methanol to 10 mL; each of the pesticides is dissolved with acetone and then diluted with methanol to 10 mL; each of the diaveridine, albendazole, albendazole sulfone and febantel is dissolved with formic acid and diluted with methanol to 10 mL; and the rest substances are dissolved with a mixed solvent of methanol and ammonia water or a mixed solvent of methanol and dimethyl sulfoxide, and then diluted with methanol to 10 mL; (2) pipetting 100 μL of the stock solution of each reference standard in the same category in Table 1 to a volumetric flask followed by diluting with methanol to 10 mL to accordingly prepare 16 first mixed standard solutions, wherein a concentration of each reference standard in the corresponding first mixed standard solution thereof is g/mL; pipetting 100 μL of each first mixed standard solution to another volumetric flask followed by diluting with methanol to 10 mL to prepare a second mixed standard solution, wherein each reference standard in the second mixed standard solution has a concentration of 100 ng/mL; and pipetting 100 μL, 500 μL, 1 mL and 5 mL, respectively, to a volumetric flask followed by diluting with a 20% aqueous methanol solution to 10 mL to prepare a series of mixed standard working solutions, wherein each reference standard in the series of mixed standard working solutions has a concentration of 1 ng/mL, 5 ng/mL, 10 ng/mL and 50 ng/mL, respectively; (3) adding 5 g of a sample to a 50 mL centrifuge tube; adding an extracting solution followed by vortex mixing; shaking and centrifuging the centrifuge tube; transferring a first supernatant to another 50 mL centrifuge tube; if the sample is muscle or poultry egg, treating a precipitate with the extracting solution again to collect an extract; combining the first supernatant with the extract to produce a mixture; adding 6 g of anhydrous sodium sulfate and 1.5 g of sodium chloride to the mixture followed by vortex mixing for 30 s and standing; and centrifuging the another 50 mL centrifuge tube to produce a second supernatant; if the sample is cow's milk or goat's milk, adding 4 g of anhydrous sodium sulfate and 1 g of sodium chloride to the first supernatant followed by vortex mixing for 30 s and standing; and centrifuging the another 50 mL centrifuge tube to produce a second supernatant; (4) activating and equilibrating a solid phase extraction column sequentially with 4 mL of methanol and 4 mL of water; rinsing the solid phase extraction column with 2 mL of the second supernatant and discarding a first effluent; loading 5 mL of the second supernatant to the solid phase extraction column and collecting a second effluent with a centrifuge tube; drying 4 mL of the second effluent in nitrogen flow at 40° C.; adding 200 μL of methanol to the dried product followed by vortex mixing for 10 s; adding 800 μL of pure water followed by vortex mixing for 30 s to produce a sample solution; and filtering the sample solution with 0.22 μm nylon filter membrane and collecting a filtrate for use; (5) diluting each of the first mixed standard solutions such that each reference standard in the corresponding diluted first mixed standard solution thereof has a concentration of 1 μg/mL; subjecting each of the diluted first mixed standard solutions to full scan and secondary scan in positive and negative modes using a ultra high performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometer; analyzing the scan results to obtain information of each reference standard using Trace Finder software of Q-Exactive plus; and establishing a mass spectrometry database based on the information of each reference standard; wherein the information of each reference standard comprises retention time, precise molecular weight of parent ion, addition mode, fragment ions, isotope abundance ratio and a fragment ion mass spectrometry generated through the superposition of fragment ion mass spectrometries obtained at collision energies of 20 eV, 40 eV and 60 eV; the isomerides are further identified through the analysis of single standard reference; and the mass spectrometry database is shown in Table 1; (6) analyzing the filtrate by the ultra high performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometer to obtain original data of the sample; (7) subjecting the original data of the sample to automatic extraction using Trace Finder software within a mass window of 5 ppm; wherein when a compound meets the following conditions at the same time, it indicates that the compound is a suspected positive compound in the sample: (i) a signal-noise ratio (S/N) of the compound is greater than 3; (ii) a difference between the measured retention time of the compound and the retention time of the compound in the mass spectrometry database is equal to or less than 0.2 min or within ±2.5% (not to exceed 0.5 min); (iii) the measured parent ion and one fragment ion of the compound simultaneously match the information in the mass spectrometry database, and a mass accuracy of the parent ion is less than 5 ppm and a mass accuracy of the fragment ion is less than 10 ppm; and (8) comparing a fragment ion mass spectrometry of the suspected positive compound in the sample with a fragment ion mass spectrometry of standard for further identification; wherein if the mass accuracy between main fragment ions in the two fragment ion mass spectrometry is less than 10 ppm and the main fragment ions in the two fragment ion mass spectrometries have identical relative abundance, it confirms that the suspected positive compound is present in the sample. In an embodiment, in step (3), when the sample is muscle or poultry egg, the extracting solution is a mixed solution of 3.0 mL of Mcllvaine-Na2EDTA buffered solution and 10 mL of acetonitrile, where a concentration of EDTA in the Mcllvaine-Na2EDTA buffered solution is 0.1 mol/L; when the sample is cow's milk or goat's milk, the extracting solution is 20 mL of acetonitrile. In an embodiment, in step (4), the solid phase extraction column is a HyperSep Retain PEP column. In an embodiment, in steps (5) and (6), parameters of the ultra high performance liquid chromatography are set as follows: chromatographic column: C18 (150 mm×3.0 mm, particle size 1.8 m); column temperature: 30° C.; injection volume: 10 μL; flow rate: 0.3 mL/min; mobile phase A: 0.1% aqueous formic acid solution for positive ion mode and 0.03% aqueous ammonia solution for negative ion mode; mobile phase B: a solution of 0.1% formic acid in acetonitrile for positive ion mode and a solution of 0.03% ammonia in acetonitrile for negative ion mode; a gradient elution program in positive ion mode is shown in Table 2, and a gradient elution program in negative ion mode is shown in Table 3. In an embodiment, in steps (5) and (6), parameters of the mass spectrometry are set as follows: ion source: electron spray ion source (ESI+ and ESI−); scan mode: full scan and automatically-triggered secondary scan mode; capillary voltage: 3.2 kv (ESI+) and 2.8 kv (ESI−); ion transmission capillary temperature: 325° C.; atomizing gas temperature: 350° C.; sheath gas pressure: 40 arb; auxiliary gas pressure: 40 arb; dwell time of the primary mass spectrometry: 100 ms; dwell time of the secondary mass spectrometry: 60 ms; scanning range (m/z): 50-1000; and collision energy: 20, 40 and 60 eV. In an embodiment, in step (3), the shaking is performed at 6,000 rpm for 10 min; and the centrifugation is performed at 4,000-7,000 rpm for 5 min. Compared with the prior art, the invention has the following beneficial effects. 1. The invention facilitates collecting high-quality and high-accuracy data, reducing the false positive rate. 2. The sample is directly analyzed in full scan mode, and there is no limit to the number of compounds scanned per unit time. Moreover, it is convenient to establish a method for the instrumental analysis. 3. The invention enables the rapid screening of compounds in the sample by comparing the measured data with the mass spectrometry database, and is particularly suitable for the screening and confirmation of multiple types of compounds. 4. The invention is suitable for the detection of veterinary drugs and additives in animal-derived food, which enriches the detection and screening technical means for multiple residues in animal-derived food, facilitating ensuring the safety of animal husbandry and food industry. Provided herein was a screening and confirmation method for veterinary drugs and additives in animal-derived food, where the veterinary drugs and additives were selected from: (a) sulfonamides: sulfamethazine, sulformethoxine, sulfisoxazole, sulfaguanidine, sulfamerazine, sulfamethylthiadizaole, sulfamethoxazole, sulfamethoxydiazine, sulfamethoxypyridazine, sulfamonomethoxine, sulfamethazole, sulfapyridinee, sulfaquinoxaline, sulfathiazole, sulfisomidine, trimethoprim, diaveridine, phthalylsulfathiazole, sulfabenzamide, sulfacetamide, sulfachlorpyridazine, sulfachloropyrazine, sulfadiazine and sulfadimethoxine; (b) fluoroquinolones: lomefloxacin, marbofloxacin, nadifloxacin, nalidixic acid, norfloxacin, ofloxacin, orbifloxacin, oxolinic acid, pipemidic acid, pefloxacin, cinoxacin, sarafloxacin, sparfloxacin, ciprofloxacin, danofloxacin, difloxacin, enoxacin, enrofloxacin, fleroxacin, flumequine and gatifloxacin; (c) benzimidazoles: mebendazole, mebendazole-amine, 2-aminofluorobenzimidazole, oxfendazole, oxibendazole, parbendazole, 5-hydroxythiabendazole, tiabendazole, albendazole, albendazole sulfone, albendazole sulfoxide, amino albendazole sulfone, benzimidazole, febantel and lobendazole; (d) stimulants: cyproheptadine, fenoterol, labetalol, penbutolol, phenylethanolamine A, ractopamine, salbutamol, salmeterol, terbutaline, mabuterol, tulobuterol, bambuterol, brombuterol, cimaterol, cimbuterol, clenbuterol, clenproperol and clonidine; (e) hormones: chlormadinone acetate, 17a-methyltestosterone, clobetasol propionate, clobetasone butyrate, corticosterone, hydrocortisone, cortisone acetate, cortisone, deflazacort, dexamethasone, diflorasone diacetate, epitestosterone, estradiol benzoate, fludrocortisone acetate, flumetasone pivalate, flumethasone, fluocinolone acetonide, fluorometholone, flurandrenolide, fluticasone propionate, nandrolone propionate, halcinonide, dihydrodiethylstilbestrol, medroxyprogesterone acetate, megestrol acetate, melengestrol acetate, methandienone, methylprednisolone, norethindrone, nandrolone propionate, levonorgestrel, prednicarbate, prednisolone, prednisone, progesterone, dehydrotestosterone, testosterone, trenbolone, triamcinolone acetonide, beclomethasone, betamethasone dipropionate, betamethasone valerate and betamethasone; (f) nitroimidazoles: dimetridazole, ipronidazole, metronidazole, ornidazole, hydroxydimetridazole, ronidazole, secnidazole and tinidazole; (g) antiviral agents: amantadine and rimantadine; (h) lincosamide: clindamycin and lincomycin; (i) amphenicols: chloramphenicol, florfenicol and thiamphenicol; (j) penicillins: ceftiofur, flucloxacillin, cloxacillin, nafcillin, piperacillin and penicillin G; (k) quinoxalines: quinocetone; (l) antipyretic and analgesics: phenacetin, salicylic acid, amidopyrine, antipyrine, dapsone, flunixin meglumine and acetaminophen; (m) psychoactive agents: haloperidol, imipramine, nitrazepam, oxazepam, pemoline, perphenazine, promethazine, propionylpromazine hydrochloride, sulpiride, xylazine hydrochloride, acepromazin, anisopirol, azaperone, carbamazepine, chloropromazine, diazepam, diphenhydramine hydrochloride, droperidol, estazolam, clarithromycin, tiamulin, erythromycin, kitasamycin, tilmicosin and tylosin; (n) anthelmintics: toltrazuril sulfocide, clopidol, dinitolmide, halofuginone and levamisole; (o) pesticides: buprofezin, carboxin, 3-hydroxycarbofuran, clothianidin, cyromazine, diuron, ethopabate, fipronil, fluridone, acephate, hexazinone, imazalil, imidacloprid, linuron, metribuzin, myclobutanil, acetamiprid, norflurazon, propyzamide, atrazine, simazine, desethyl atrazine, azoxystrobin and benoxacor; and (p) other substances: scopolamine, valnemulin and atropine. The method was specifically described as follows. 1.1 Preparation of Stock Solutions 10 mg of each reference standard was weighed accurately, dissolved and diluted with methanol to 10 mL in a volumetric flask to prepare a 1 mg/mL stock solution of each reference standard; where norfloxacin was dissolved with water; the pesticides were dissolved with acetone; diaveridine, albendazole, albendazole sulfone and febantel were all dissolved with formic acid and then diluted with methanol to 10 mL; and the rest substances with lower solubility were dissolved with a mixed solvent of methanol and ammonia water or a mixed solvent of methanol (main solvent) and dimethyl sulfoxide (auxiliary solvent), and then diluted to 10 mL. 1.2 Preparation of Standard Working Solutions (1) 100 μL of the stock solution of each reference standard in the same category in Table 1 was accurately pipetted to a volumetric flask, and then diluted to 10 mL with methanol to accordingly prepare 16 first mixed standard solutions, where each reference standard in the corresponding first mixed standard solution thereof had a concentration of 10 μg/mL. 100 μL of each first mixed standard solution was accurately pipetted to another volumetric flask and then diluted to 10 mL with methanol to prepare a second mixed standard solution, where each reference standard in the second mixed standard solution had a concentration of 100 ng/mL. (2) accurately 100 μL, 500 μL, 1 mL and 5 mL of the second mixed standard solution were pipetted, respectively, and, then diluted to 10 mL with a 20% aqueous methanol solution to prepare a series of mixed standard working solutions, where each reference standard in the series of mixed standard working solutions had a concentration of 1 ng/mL, 5 ng/mL, 10 ng/mL and 50 ng/mL, respectively. 2.1 Sample Preparation 2.1.1 Animal Tissues An appropriate amount of fresh/thawed blank or test tissue was collected, minced and homogenized. 2.1.2 Cow's Milk and Goat's Milk An appropriate amount of fresh/thawed blank or test cow's milk was collected and mixed uniformly. 2.1.3 Poultry Eggs An appropriate number of fresh test eggs were collected, shelled and homogenized. The above samples were all stored below −20° C. 2.2 Extraction 2.2.1 Muscle or Poultry Egg 5 g of the sample was added into a 50 mL centrifuge tube, to which a mixed solution of 3.0 mL Mcllvaine-Na2EDTA buffered solution and 10 mL acetonitrile was added for extraction, where the Mcllvaine-Na2EDTA buffered solution was prepared through steps of: dissolving 44.08 g of disodium hydrogen phosphate, 37.2 g of Na2EDTA and 8.08 g of citric acid followed by diluting to 1000 mL to prepare 0.1 mol/L Mcllvaine-Na2EDTA buffered solution. The centrifuge tube was subjected to vortex mixing, shook at 6,000 rpm for 10 min and centrifuged at 4,000 rpm for 5 min. The supernatant was transferred to another 50 mL centrifuge tube, and the precipitate was repeatedly treated with the extraction solution. The two supernatants were combined, added with 6 g of anhydrous sodium sulfate and 1.5 g of sodium chloride, mixed under vortex for 30 s, subjected to standing for 10 min and centrifuged at 7,000 rpm for 5 min, and the supernatant was collected for further use. 2.2.2 Cow's Milk or Goat's Milk 5 g of cow's milk or goat's milk was added into a 50 mL centrifuge tube, to which 20 mL of acetonitrile was added for extraction. The centrifuge tube was subjected to vortex mixing, shook at 6,000 rpm for 10 min and centrifuged at 7,000 rpm for 5 min, and the supernatant was transferred to another 50 mL centrifuge tube, added with 4 g of anhydrous sodium sulfate and 1 g of sodium chloride, mixed under vortex for 30 s, subjected to standing for 10 min and centrifuged at 7,000 rpm for 5 min. The supernatant was collected for further use. 2.3 Purification The solid phase extraction column was activated and equilibraed sequentially with 4 mL of methanol and 4 mL of water and then rinsed with 2 mL of the supernatant prepared in step (2.2.1) or (2.2.2). The effluent was discarded, and then 5 mL of the supernatant was loaded to the solid phase extraction column. The effluent was collected by a centrifuge tube, and 4 mL of the effluent was accurately pipetted, dried at 40° C. in nitrogen flow, added with 200 μL of methanol, mixed under vortex for 10 s, added with 800 μL of pure water, mixed under vortex for 30 s and filtered through a 0.22 m nylon filter membrane. The filtrate was collected and stored for further use. 3.1 Liquid Chromatography The gradient elution programs in positive and negative ion modes were respectively shown in Table 4 and Table 5. 3.2 Mass Spectrometry Parameters of the mass spectrometry were set as follows: ion source: electron spray ion source (ESI+ and ESI−); capillary voltage: 3.2 kv (ESI+), 2.8 kv (ESI−); ion transmission capillary temperature: 325° C.; atomizing gas temperature: 350° C.; sheath gas pressure: 40 arb; auxiliary gas pressure: 40 arb; dwell time of the primary mass spectrometry: 100 ms; dwell time of the secondary mass spectrometry: 60 ms; scanning range (m/z): 50-1000; collision energy: 20, 40, 60 eV; scan mode: full scan and automatically-triggered secondary scan mode. The 16 first mixed standard solutions were diluted such that each reference standard in the corresponding diluted first mixed standard solution thereof had a concentration of 1 μg/mL. Each of the diluted first mixed standard solutions was subjected to full scan and secondary scan in the positive and negative modes using ultra high performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometer. The obtained scan results were analyzed using Trace Finder software of Q-Exactive plus to obtain information of each reference standard, where the information of each reference standard included retention time, precise molecular weight of parent ion, addition mode, fragment ions, isotope abundance ratio and a fragment ion mass spectrometry generated through the superposition of fragment ion mass spectrometries obtained at collision energies of 20 eV, 40 eV and 60 eV. A mass spectrometry database was established based on the information of each reference standard, which was shown in Table 1. Those isomerides were further identified through the analysis of single reference standard. Enrofloxacin (1 μg/mL) was used as an example, and its parent ion extraction chromatogram, parent ion and fragment ion mass spectrometry were shown in Table 1 Mass spectrometry library information The filtrate obtained in step (2.3) was analyzed by ultra high performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometer to obtain original data of the sample. The original data of the sample was automatically extracted within a mass window of 5 ppm using the Trace Finder software, where when a compound met the following conditions at the same time, it indicated that the compound was a suspected positive compound in the sample: (i) a signal-noise ratio (S/N) is greater than 3; (ii) a difference between the measured retention time and the corresponding retention time in the mass spectrometry database is <0.2 min or within ±2.5% (no more than 0.5 min); (iii) the measured parent ion and one fragment ion simultaneously matched the corresponding information in the mass spectrometry database, and a mass accuracy of the parent ion was less than 5 ppm and a mass accuracy of the fragment ion was less than 10 ppm. A fragment ion mass spectrometry of the suspected positive compound in the sample was compared with a fragment ion mass spectrometry of standard for further identification, where if the mass accuracy between main fragment ions in the two fragment ion mass spectrometries is less than 10 ppm and the main fragment ions in the two fragment ion mass spectrometries have identical relative abundance, it confirmed that the suspected positive compound was present in the sample. The detection limit of each compound of the veterinary drugs and additives in the invention was the lowest concentration that the compound can be detected in the sample in the screening in step (6) and in the confirmation in step (7). Further, the detection limits of the veterinary drugs and additives were shown in Table 6. The parent ion extraction chromatograms of reference standards of the veterinary drugs and additives were shown in It can be seen from above that the method of the invention mainly achieved the screening and confirmation of compounds, that was, this method was suitable for the qualification of the residues in the animal-derived food. Therefore, the validity of the method of the invention was assessed mainly in terms of detection limit, recovery rate, precision and matrix effect. 9.1 Detection Limit The blank sample was processed in the same manner and then analyzed. The measurement results showed that the blank sample has no interference with the drug to be tested at the corresponding retention time. If the blank sample had a peak at the retention time of the drug to be tested, the value of the blank sample should be deducted before the calculation. An appropriate amount of the mixed standard solution was added into 5 g of the blank matrix, which was then processed by the above pretreatment method and analyzed. The detection limit of each compound in chicken, pork, mutton, beef, chicken eggs, duck eggs, cow's milk and goat's milk was determined according to the conditions of the compound confirmation. The results were shown in Table 6. 9.2 Recovery Rate and Precision A standard solution having a concentration near the detection limit was added to the blank sample according to the spiking method. 5 parallel samples of each concentration were tested 3 times for the calculation of intra- and inter-batch relative standard deviations (RSD). The results were shown in Table 6, in which the recovery rates of the 207 veterinary drugs and additives were all in the range of 40%-130%, and intra- and inter-batch relative standard deviations were less than 30%. 9.3 Matrix Effect The matrix effect of the sample was evaluated by comparing the standard solution and the spiked sample liquid before the nitrogen blowing in the chromatographic peak area. The results showed that the substances in the matrix may show matrix inhibition or matrix enhancement, where a matrix effect value between 0.85 and 1.15 indicated that there was no significant matrix effect; a matrix effect value greater than 1.15 indicated matrix enhancement; and a matrix effect value less than 0.85 indicated matrix inhibition. The matrix effect values measured herein ranged from 0.11 to 8.03, therefore, it was required to plot a matrix-matched standard curve for the quantification. Described above are merely preferred embodiments of the invention. It should be noted that any modification and change made by those skilled in the art without departing from the spirit of the invention should fall within the scope of the invention. A screening and confirmation method for veterinary drugs and additives in animal-derived food, including: preparation of standard working solution, pretreatment of samples to be tested, establishment of database, sample detection, compound screening and compound confirmation. The disclosure employs ultra high performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometry, and enables the simultaneous detection of 207 veterinary drugs and additives in animal-derived food within 22 min. 1. A screening and confirmation method for veterinary drugs and additives in animal-derived food, comprising:
(1) dissolving 10 mg of each reference standard of the veterinary drugs and additives followed by diluting to 10 mL in a volumetric flask to prepare a 1 mg/mL stock solution of each reference standard; wherein the veterinary drugs and additives are selected from: (a) sulfonamides: sulfamethazine, sulformethoxine, sulfisoxazole, sulfaguanidine, sulfamerazine, sulfamethylthiadizaole, sulfamethoxazole, sulfamethoxydiazine, sulfamethoxypyridazine, sulfamonomethoxine, sulfamethazole, sulfapyridinee, sulfaquinoxaline, sulfathiazole, sulfisomidine, trimethoprim, diaveridine, phthalylsulfathiazole, sulfabenzamide, sulfacetamide, sulfachlorpyridazine, sulfachloropyrazine, sulfadiazine and sulfadimethoxine; (b) fluoroquinolones: lomefloxacin, marbofloxacin, nadifloxacin, nalidixic acid, norfloxacin, ofloxacin, orbifloxacin, oxolinic acid, pipemidic acid, pefloxacin, cinoxacin, sarafloxacin, sparfloxacin, ciprofloxacin, danofloxacin, difloxacin, enoxacin, enrofloxacin, fleroxacin, flumequine and gatifloxacin; (c) benzimidazoles: mebendazole, mebendazole-amine, 2-aminofluorobenzimidazole, oxfendazole, oxibendazole, parbendazole, 5-hydroxythiabendazole, tiabendazole, albendazole, albendazole sulfone, albendazole sulfoxide, amino albendazole sulfone, benzimidazole, febantel and lobendazole; (d) stimulants: cyproheptadine, fenoterol, labetalol, penbutolol, phenylethanolamine A, ractopamine, salbutamol, salmeterol, terbutaline, mabuterol, tulobuterol, bambuterol, brombuterol, cimaterol, cimbuterol, clenbuterol, clenproperol and clonidine; (e) hormones: chlormadinone acetate, 17a-methyltestosterone, clobetasol propionate, clobetasone butyrate, corticosterone, hydrocortisone, cortisone acetate, cortisone, deflazacort, dexamethasone, diflorasone diacetate, epitestosterone, estradiol benzoate, fludrocortisone acetate, flumetasone pivalate, flumethasone, fluocinolone acetonide, fluorometholone, flurandrenolide, fluticasone propionate, nandrolone propionate, halcinonide, dihydrodiethylstilbestrol, medroxyprogesterone acetate, megestrol acetate, melengestrol acetate, methandienone, methylprednisolone, norethindrone, nandrolone propionate, levonorgestrel, prednicarbate, prednisolone, prednisone, progesterone, dehydrotestosterone, testosterone, trenbolone, triamcinolone acetonide, beclomethasone, betamethasone dipropionate, betamethasone valerate and betamethasone; (f) nitroimidazoles: dimetridazole, ipronidazole, metronidazole, ornidazole, hydroxydimetridazole, ronidazole, secnidazole and tinidazole; (g) antiviral drugs: amantadine and rimantadine; (h) lincosamide: clindamycin and lincomycin; (i) amphenicols: chloramphenicol, florfenicol and thiamphenicol; (j) penicillins: ceftiofur, flucloxacillin, cloxacillin, nafcillin, piperacillin and penicillin G; (k) quinoxalines: quinocetone; (l) antipyretics and analgesics: phenacetin, salicylic acid, amidopyrine, antipyrine, dapsone, flunixin meglumine and acetaminophen; (m) psychoactive drugs: haloperidol, imipramine, nitrazepam, oxazepam, pemoline, perphenazine, promethazine, propionylpromazine hydrochloride, sulpiride, xylazine, acepromazin, anisopirol, azaperone, carbamazepine, chloropromazine, diazepam, diphenhydramine, droperidol, estazolam, clarithromycin, tiamulin, erythromycin, kitasamycin, tilmicosin and tylosin; (n) anthelmintics: toltrazuril sulfoxide, clopidol, dinitolmide, halofuginone and levamisole; (o) pesticides: buprofezin, carboxin, 3-hydroxycarbofuran, clothianidin, cyromazine, diuron, ethopabate, fipronil, fluridone, acephate, hexazinone, imazalil, imidacloprid, linuron, metribuzin, myclobutanil, acetamiprid, norflurazon, propyzamide, atrazine, simazine, desethyl atrazine, azoxystrobin and benoxacor; (p) other substances: scopolamine, valnemulin and atropine; the norfloxacin is dissolved with water and diluted with methanol to 10 mL; each of the pesticides is dissolved with acetone and then diluted with methanol to 10 mL; each of the diaveridine, albendazole, albendazole sulfone and febantel is dissolved with formic acid and diluted with methanol to 10 mL; and the rest substances are dissolved with a mixed solvent of methanol and ammonia water or a mixed solvent of methanol and dimethyl sulfoxide, and then diluted with methanol to 10 mL; (2) pipetting 100 μL of the stock solution of each reference standard in the same category in Table 1 to a volumetric flask followed by diluting with methanol to 10 mL to accordingly prepare 16 first mixed standard solutions, wherein a concentration of each reference standard in the corresponding first mixed standard solution thereof is 10 μg/mL; pipetting 100 μL of each first mixed standard solution to another volumetric flask followed by diluting with methanol to 10 mL to prepare a second mixed standard solution, wherein each reference standard in the second mixed standard solution has a concentration of 100 ng/mL; and pipetting 100 μL, 500 μL, 1 mL and 5 mL, respectively, to a volumetric flask followed by diluting with a 20% aqueous methanol solution to 10 mL to prepare a series of mixed standard working solutions, wherein each reference standard in the series of mixed standard working solutions has a concentration of 1 ng/mL, 5 ng/mL, 10 ng/mL and 50 ng/mL, respectively; (3) adding 5 g of a sample to a 50 mL centrifuge tube; adding an extracting solution followed by vortex mixing; shaking and centrifuging the centrifuge tube; transferring a first supernatant to another 50 mL centrifuge tube; if the sample is muscle or poultry egg, treating a precipitate with the extracting solution again to collect an extract; combining the first supernatant with the extract to produce a mixture; adding 6 g of anhydrous sodium sulfate and 1.5 g of sodium chloride to the mixture followed by vortex mixing for 30 s and standing; and centrifuging the another 50 mL centrifuge tube to produce a second supernatant; if the sample is cow's milk or goat's milk, adding 4 g of anhydrous sodium sulfate and 1 g of sodium chloride to the first supernatant followed by vortex mixing for 30 s and standing; and centrifuging the another 50 mL centrifuge tube to produce a second supernatant; (4) activating and equilibrating a solid phase extraction column sequentially with 4 mL of methanol and 4 mL of water; rinsing the solid phase extraction column with 2 mL of the second supernatant and discarding a first effluent; loading 5 mL of the second supernatant to the solid phase extraction column and collecting a second effluent with a centrifuge tube; drying 4 mL of the second effluent in nitrogen flow at 40° C.; adding 200 μL of methanol to the dried product followed by vortex mixing for 10 s; adding 800 μL of pure water followed by vortex mixing for 30 s to produce a sample solution; and filtering the sample solution with 0.22 m nylon filter membrane and collecting a filtrate for use; (5) diluting each of the first mixed standard solutions such that each reference standard in the corresponding diluted first mixed standard solution thereof has a concentration of 1 μg/mL; subjecting each of the diluted first mixed standard solutions to full scan and secondary scan in positive and negative modes using a ultra high performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometer; analyzing the scan results to obtain information of each reference standard through Trace Finder software of Q-Exactive plus; and establishing a mass spectrometry database based on the information of each reference standard; wherein the information of each reference standard comprises retention time, precise molecular weight of parent ion, addition mode, fragment ions, isotope abundance ratio and a fragment ion mass spectrometry generated through the superposition of fragment ion mass spectrometry obtained at collision energies of 20 eV, 40 eV and 60 eV; the isomeride are further identified through the analysis of single standard reference; and the mass spectrometry database is shown in Table 1; (6) analyzing the filtrate by the ultra high performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometer to obtain original data of the sample; (7) subjecting the original data of the sample to automatic extraction using Trace Finder software within a mass window of 5 ppm; wherein when a compound meets the following conditions at the same time, it indicates that the compound is a suspected positive compound in the sample: (i) a signal-noise ratio (S/N) of the compound is greater than 3; (ii) a difference between the measured retention time of the compound and the retention time of the compound in the mass spectrometry database is equal to or less than 0.2 min or within ±2.5% (not to exceed 0.5 min); (iii) the measured parent ion and one fragment ion of the compound simultaneously match the information in the mass spectrometry database, and a mass accuracy of the parent ion is less than 5 ppm and a mass accuracy of the fragment ion is less than 10 ppm; and (8) comparing a fragment ion mass spectrometry of the suspected positive compound in the sample with a fragmentation mass spectrometry of standard for further identification; wherein if the mass accuracy between main fragment ions in the two fragment ion mass spectrometries is less than 10 ppm and the main fragment ions in the two fragment ion mass spectrometries have identical relative abundance, it confirms that the suspected positive compound is present in the sample. 2. The screening and confirmation method of 3. The screening and confirmation method of 4. The screening and confirmation method of chromatographic column: C18 (150 mm×3.0 mm, particle size 1.8 μm); column temperature: 30° C.; injection volume: 10 μL; flow rate: 0.3 mL/min; mobile phase A: 0.1% aqueous formic acid solution for positive ion mode and 0.03% aqueous ammonia solution for negative ion mode; mobile phase B: a solution of 0.1% formic acid in acetonitrile for positive ion mode and a solution of 0.03% ammonia in acetonitrile for negative ion mode; a gradient elution program in positive ion mode is shown in Table 2, and a gradient elution program in negative ion mode is shown in Table 3. 5. The screening and confirmation method of ion source: electron spray ion source (ESI+ and ESI−); scan mode: full scan and automatically triggered secondary scan mode; capillary voltage: 3.2 kv (ESI+) and 2.8 kv (ESI−); ion transmission capillary temperature: 325° C.; atomizing gas temperature: 350° C.; sheath gas pressure: 40 arb; auxiliary gas pressure: 40 arb; dwell time of the primary mass spectrometry: 100 ms; dwell time of the secondary mass spectrometry: 60 ms; scanning range (m/z): 50-1000; and collision energy: 20, 40 and 60 eV. 6. The screening and confirmation method of CROSS-REFERENCE TO RELATED APPLICATIONS
TECHNICAL FIELD
BACKGROUND
SUMMARY
Mass spectrometry library information Molecular Addition Parent Retention Category Compound formula mode ion Fragment 1 Fragment 2 time (min) Sulfamethazine C12H14N4O2S M + H 279.09102 156.01138 124.08694 8.44 Sulformethoxine C12H14N4O4S M + H 311.08085 156.01138 140.04545 9.55 Sulfisoxazole C11H13N3O3S M + H 268.07504 156.0111 108.04462 9.83 Sulfaguanidine C7H10N4O2S M + H 215.05972 156.01138 108.04439 3.43 Sulfamerazine C11H12N4O2S M + H 265.07537 190.02809 156.01138 7.89 Sulfamethylthiadizaole C9H10N4O2S2 M + H 271.03179 156.01138 108.04439 8.31 Sulfamethoxazole C10H11N3O3S M + H 254.05939 156.01138 108.04439 9.57 Sulfamethoxydiazine C11H12N4O3S M + H 281.07029 108.04465 156.01115 8.53 Sulfamethoxypyridazine C11H12N4O3S M + H 281.07029 215.09274 156.01138 8.41 Sulfamonomethoxine C11H12N4O3S M + H 281.07029 215.09274 156.01138 8.9 Sulfonamides Sulfamethazole C11H13N3O3S M + H 268.07504 156.0111 108.04462 8.03 Sulfapyridinee C11H11N3O2S M + H 250.06447 184.08692 156.01138 7.55 Sulfaquinoxaline C14H12N4O2S M + H 301.07537 156.01138 108.04439 10.39 Sulfathiazole C9H9N3O2S2 M + H 256.02089 156.01138 108.04439 7.28 Sulfisomidine C12H14N4O2S M + H 279.09102 124.08692 108.04439 6.57 Trimethoprim C14H18N4O3 M + H 291.14517 275.11387 261.09822 7.28 Diaveridine C13H16N4O2 M + H 261.1346 245.1033 123.06652 7 Phthalylsulfathiazole C17H13N3O5S2 M + H 404.03694 149.02313 256.02032 9.07 Sulfabenzamide C13H12N2O3S M + H 277.06414 156.01138 108.04439 10.32 Sulfacetamide C8H10N2O3S M + H 215.04849 173.03792 156.01138 6.73 Sulfachlorpyridazine C10H9ClN4O2S M + H 285.02075 156.01138 108.04439 9.25 Sulfachloropyrazine C10H9ClN4O2S M + H 285.02075 130.0166 156.01111 10.35 Sulfadiazine C10H10N4O2S M + H 251.05972 156.01138 108.04439 7.13 Sulfadimethoxine C12H14N4O4S M + H 311.08085 156.07675 108.04439 10.43 Fluoroquinolones Lomefloxacin C17H19F2N3O3 M + H 352.14672 308.1569 265.1147 7.58 Marbofloxacin C17H19FN4O4 M + H 363.14631 320.10411 72.08078 7.17 Nadifloxacin C19H21FN2O4 M + H 361.15581 343.14389 283.08725 10.76 Nalidixic acid C12H12N2O3 M + H 233.09207 205.06077 187.0502 11.43 Norfloxacin C16H18FN3O3 M + H 320.1405 276.15067 233.10847 7.24 Ofloxacin C18H20FN3O4 M + H 362.15106 318.16123 261.10338 7.31 Orbifloxacin C19H20F3N3O3 M + H 396.15295 352.16312 295.10527 7.89 Oxolinic acid C13H11NO5 M + H 262.071 244.06043 234.0397 9.95 Pipemidic acid C14H17N5O3 M + H 304.14042 290.16632 233.10847 6.75 Pefloxacin C17H20FN3O3 M + H 334.15615 217.10839 189.0779 7.35 Cinoxacin C12H10N2O5 M + H 263.06625 235.07133 217.06077 9.47 Sarafloxacin C20H17F2N3O3 M + H 386.13107 342.14125 299.09905 8.16 Sparfloxacin C19H22F2N4O3 M + H 393.17327 349.18344 292.12559 8.22 Ciprofloxacin C17H18FN3O3 M + H 332.1405 288.15067 245.10847 7.37 Danofloxacin C19H20FN3O3 M + H 358.15615 314.16632 283.12412 7.54 Difloxacin C21H19F2N3O3 M + H 400.14672 356.15689 299.09905 8.27 Enoxacin C15H17FN4O3 M + H 321.13575 277.14592 257.13969 7.13 Enrofloxacin C19H22FN3O3 M + H 360.1718 316.18197 245.10847 7.73 Fleroxacin C17H18F3N3O M + H 370.1373 326.14747 269.08962 7.36 Flumequine C14H12FNO3 M + H 262.0874 244.07683 220.04045 11.59 Gatifloxacin C19H22FN3O4 M + H 376.16671 332.17688 289.13468 7.97 Stimulants Cyproheptadine C21H21N M + H 288.17468 191.08553 96.08078 10.67 Fenoterol C17H21NO4 M + H 304.15433 286.14377 135.08044 6.57 Labetalol C19H24N2O3 M + H 329.18597 311.1754 294.14886 8.97 Penbutolol C18H29NO2 M + H 292.22711 236.16451 201.12739 11.08 Phenylethanolamine A C19H24N2O4 M + H 345.18088 327.17032 150.09134 10.03 Ractopamine C18H23NO3 M + H 302.17507 284.16451 164.10699 7.56 Salbutamol C13H21NO3 M + H 240.15942 148.07569 166.08625 5.75 Salmeterol C25H37NO4 M + H 416.27954 398.26897 380.2584 11.03 Terbutaline C12H19NO3 M + H 226.14377 170.08117 152.07061 5.71 Mabuterol C13H18ClF3N2O M + H 311.11325 237.03951 217.03343 9 Tulobuterol C12H18ClNO M + H 228.11497 172.05237 154.0418 8.25 Bambuterol C18H29N3O5 M + H 368.218 312.1554 294.14483 8.65 Brombuterol C12H18Br2N2O M + H 364.98586 290.9127 211.99436 8.71 Cimaterol C12H17N3O M + H 220.14444 202.13387 160.08692 5.93 Cimbuterol C13H19N3O M + H 234.16009 160.08656 143.06007 6.61 Clenbuterol C12H18Cl2N2O M + H 277.0869 259.07633 203.01373 8.31 Clenproperol C11H16Cl2N2O M + H 263.07125 245.06068 203.01373 7.85 Clonidine C9H9Cl2N3 M + H 230.02463 212.99808 / 6.77 Hormones Chlormadinone acetate C23H29ClO4 M + H 405.18271 309.1849 267.1743 15.67 17a-methyltestosterone C20H30O2 M + H 303.23186 285.22129 267.21073 13.63 Clobetasol propionate C25H32ClFO5 M + H 467.19951 263.143 147.0804 15.08 Clobetasone butyrate C26H32ClFO5 M + H 479.19951 279.138 343.1459 16.13 Corticosterone C21H30O4 M + H 347.22169 329.21112 311.20056 11.61 Hydrocortisone C21H30O5 M + H 363.2166 327.19547 309.18491 10.34 Cortisone acetate C23H30O6 M + H 403.21152 343.19038 163.11174 12.53 Cortisone C21H28O5 M + H 361.20095 163.1117 121.0648 10.45 Deflazacort C25H31NO6 M + H 442.22241 142.0499 312.1958 12.39 Dexamethasone C22H29FO5 M + H 393.20718 355.19039 337.17982 11.21 Diflorasone diacetate C26H32F2O7 M + H 495.21889 121.0648 317.1536 13.91 Epitestosterone C19H28O2 M + H 289.21621 97.0648 109.0648 13.93 Estradiol benzoate C25H28O3 M + H 377.21112 279.1743 321.1849 12.22 Fludrocortisone acetate C23H31FO6 M + H 423.21774 181.1023 239.143 12.22 Flumetasone pivalate C27H36F2O6 M + H 495.25527 57.0699 121.0648 15.25 Flumethasone C22H28F2O5 M + H 411.19776 235.1117 277.1587 11.2 Fluocinolone acetonide C24H30F2O6 M + H 453.20832 121.0648 337.1434 11.86 Fluorometholone C22H29FO4 M + H 377.21226 279.1743 135.0804 12.22 Flurandrenolide C24H33FO6 M + H 437.23339 361.1798 105.0699 12.01 Fluticasone propionate C25H31F3O5S M + H 501.19171 121.0648 275.143 15.08 Nandrolone propionate C21H30O3 M + H 331.22677 257.18999 239.17943 17.38 Halcinonide C24H32ClFO5 M + H 455.19951 121.0648 377.1514 14.98 Dihydrodiethylstilbestrol C18H22O2 M + H 271.16926 109.1117 253.1587 11.92 Medroxyprogesterone C24H34O4 M + H 387.25299 123.0804 327.2319 15.81 acetate Megestrol acetate C24H32O4 M + H 385.23734 267.1743 224.156 15.53 Melengestrol acetate C25H32O4 M + H 397.23734 279.1743 337.2162 15.82 Methandienone C20H28O2 M + H 301.21621 283.20564 149.13248 12.73 Methylprednisolone C22H30O5 M + H 375.2166 161.0961 135.0804 10.99 Norethindrone C20H26O2 M + H 299.20056 109.0648 281.19 13.12 Nandrolone propionate C18H26O2 M + H 275.20056 109.06499 239.17885 12.47 Levonorgestrel C21H28O2 M + H 313.21621 109.0648 245.19 14.23 Prednicarbate C27H36O8 M + H 489.24829 147.0804 289.1587 14.91 Prednisolone C21H28O5 M + H 361.20095 147.0804 171.0804 10.2 Prednisone C21H26O5 M + H 359.1853 341.17474 237.12739 10.27 Progesterone C21H30O2 M + H 315.23186 109.06479 97.06479 15.76 Dehydrotestosterone C19H26O2 M + H 287.20056 121.0647 135.1167 12.22 Testosterone C19H28O2 M + H 289.21621 109.06479 97.06479 13.03 Trenbolone C18H22O2 M + H 271.16926 253.15869 199.11174 11.92 Triamcinolone acetonide C24H31FO6 M + H 435.21774 397.201 339.1591 11.69 Beclomethasone C22H29ClO5 M + H 409.17763 391.1671 279.1743 11.45 Betamethasone dipropionate C28H37FO7 M + H 505.25961 279.1741 319.1693 15.45 Betamethasone valerate C27H37FO6 M + H 477.26469 279.1743 147.0804 14.55 Betamethasone C22H29FO5 M + H 393.20718 355.19039 279.17434 11.14 Nitroimidazoles Dimetridazole C5H7N3O2 M + H 142.0611 112.06311 95.06037 7.17 Ipronidazole C7H11N3O2 M + H 170.0924 140.09441 123.09167 10.33 Metronidazole C6H9N3O3 M + H 172.07167 128.04545 82.05255 6.39 Omidazole C7H10ClN3O3 M + H 220.04835 128.04547 203.14304 8.77 Hydroxydimetridazole C5H7N3O3 M + H 158.05602 140.0455 110.0475 6.42 Ronidazole C6H8N4O4 M + H 201.06183 140.04545 110.04746 7.03 Secnidazole C7H11N3O3 M + H 186.08732 128.0455 111.0427 7.56 Tinidazole C8H13N3O4S M + H 248.06995 154.0611 110.08384 8.12 Antiviral Amantadine C10H17N M + H 152.14338 135.11683 107.08553 7.48 drugs Rimantadine; C12H21N M + H 180.17468 163.14778 81.0704 9 Lincosamides Clindamycin C18H33ClN2O5S M + H 425.18715 126.12773 / 8.69 Lincomycin C18H34N2O6S M + H 407.221 126.12773 359.21766 6.76 Amphenicols Chloramphenicol C11H12Cl2N2O5 M − H 321.00505 257.03346 194.04588 10.11 Florfenicol C12H14Cl2FNO4S M − H 355.99319 335.98696 185.02779 9.96 Thiamphenicol C12H15Cl2NO5S M − H 353.99752 290.02593 185.02779 8.27 β-Lactams Ceftiofur C19H17N5O7S3 M + H 524.03629 241.03899 210.02065 9.63 Flucioxacillin C19H17ClFN3O5S M + H 454.06342 160.04242 114.03742 12.5 Cioxacillin C19H18ClN3O5S M + H 436.07285 277.0374 206.0367 12.26 Nafcillin C21H22N2O5S M + H 415.13222 199.0754 171.0441 12.54 Piperacillin C23H27N5O7S M + H 518.1704 143.0815 115.0502 10.16 Penicillin G C16H18N2O4S M + H 335.106 217.06414 176.07061 10.77 Quinoxalines Quinocetone C18H14N2O3 M + H 307.10772 143.06018 273.10178 11.99 Antipyretics Phenacetin C10H13NO2 M + H 180.10191 110.0602 138.09109 10.11 and Salicylic acid C7H6O3 M − H 137.02442 93.0346 65.0397 3.74 analgesics Amidopyrine C13H17N3O M + H 232.14444 113.10738 98.08418 6.46 Antipyrine C11H12N2O M + H 189.10224 147.09135 161.10696 8.39 Dapsone C12H12N2O2S M + H 249.06922 156.01122 108.04431 9.03 Flunixin meglumine C14H11F3N2O2 M + H 297.08454 279.07397 264.0505 13.66 Acetaminophen C8H9NO2 M + H 152.07061 110.06004 / 6.25 Psychoactive Haloperidol C21H23ClFNO2 M + H 376.14741 358.13685 165.07102 10.3 drugs Imipramine C19H24N2 M + H 281.20123 86.0963 236.1434 10.71 Nitrazepam C15H11N3O3 M + H 282.08732 268.08383 207.09126 11.53 Oxazepam C15H11ClN2O2 M + H 287.05818 241.05203 269.04703 11.49 Pemoline C9H8N2O2 M + H 177.06585 106.0651 79.0542 7.74 Perphenazine C21H26ClN3OS M + H 404.15579 143.11763 171.14877 10.83 Promethazine C17H20N2S M + H 285.142 198.0372 86.09643 10.34 Propionylpromazine C20H24N2OS M + H 341.16821 86.0964 58.0651 10.95 hydrochloride Sulpiride C15H23N3O4S M + H 342.1482 214.0169 112.1121 6.21 Xylazine hydrochloride C12H16N2S M + H 221.1107 90.0372 164.0528 8.27 Acepromazin C19H22N2OS M + H 327.15256 254.06341 86.09643 10.2 Anisopirol C19H24FN3O M + H 330.19762 312.1865 121.076 7.44 Azaperone C19H22FN3O M + H 328.18197 165.07069 121.07603 8.04 Carbamazepine C15H12N2O M + H 237.10224 194.0964 220.0757 11.15 Chloropromazine C17H19ClN2S M + H 319.10302 86.09643 246.01379 11.25 Diazepam C16H13ClN2O M + H 285.07892 154.0415 193.0883 13.58 Diphenhydramine C17H21NO M + H 256.16959 167.0855 152.0621 10.03 hydrochloride Droperidol C22H22FN3O2 M + H 380.17688 165.07072 194.09726 9.35 Estazolam C16H11ClN4 M + H 295.0745 267.05524 205.0755 11.44 Macrolides Clarithromycin C38H69NO13 M + H 748.48417 158.11756 116.07061 11.04 Tiamulin C28H47NO4S M + H 494.32986 192.10528 119.01613 10.8 Erythromycin C37H67NO13 M + H 734.46852 158.11756 116.07061 9.92 Kitasamycin C40H67NO14 M + H 786.46343 558.32727 174.11247 11.25 Tilmicosin C46H80N2O13 M + H 869.57332 174.11247 88.07569 8.93 Tylosin C46H77NO17 M + H 916.52643 772.44778 174.11247 10.17 anthelmintics Mebendazole C16H13N3O3 M + H 296.10297 264.07675 105.03349 10.85 Mebendazole-amine C14H11N3O M + H 238.09749 105.03369 / 8.45 2-aminofluorobenzimidazole C14H10FN3O M + H 256.08807 123.0241 113.0397 8.74 Oxfendazole C15H13N3O3S M + H 316.07504 284.04882 223.0576 9.26 Oxibendazole C12H15N3O3 M + H 250.11862 218.0924 176.04545 9.33 Parbendazole C13H17N3O2 M + H 248.13935 216.1131 160.0505 10.65 5-hydroxythiabendazole C10H7N3OS M + H 218.03826 191.02736 / 6.44 Tiabendazole C10H7N3S M + H 202.04334 175.03245 131.06037 7.12 Albendazole C12H15N3O2S M + H 266.09577 234.06956 191.01478 10.99 Albendazole sulfone C12H15N3O4S M + H 298.0856 266.05939 224.01244 9.4 Albendazole sulfoxide C12H15N3O3S M + H 282.09069 240.04347 208.01752 8.08 Amino albendazole C10H13N3O2S M + H 240.08012 198.03317 165.05328 6.94 sulfone Benzimidazole C7H6N2 M + H 119.06037 92.0495 65.0386 4.8 Febantel C20H22N4O6S M + H 447.13328 383.08085 280.05391 14.78 Lobendazole C10H11N3O2 M + H 206.0924 160.0505 178.0609 7.59 Toltrazuril sulfoxide C18H14F3N3O5S M − H 440.05335 371.05813 / 8.97 Clopidol C7H7Cl2NO M + H 191.99775 86.9996 101.01525 6.83 Dinitolmide C8H7N3O5 M − H 224.03129 181.02438 77.03858 9.91 Halofuginone C16H17BrClN3O3 M + H 414.02146 100.07598 120.08076 8.89 Levamisole C11H12N2S M + H 205.0794 178.0685 / 6.99 Pesticides Buprofezin C16H23N3OS M + H 306.16346 201.10561 116.05285 17.73 Carboxin C12H13NO2S M + H 236.07398 143.01613 93.0573 12.61 3-hydroxycarbofuran C12H15NO4 M + H 238.10738 163.07536 135.08044 9.09 Clothianidin C6H8ClN5O2S M + H 250.016 131.96692 113.0168 8.96 Cyromazine C6H10N6 M + H 167.10397 85.05087 125.08217 3.63 Diuron C9H10Cl2N2O M + H 233.02429 72.04439 159.97153 12.65 Ethopabate C12H15NO4 M + H 238.10738 206.08117 164.07061 10.29 Fipronil C12H4Cl2F6N4OS M − H 434.93143 329.95845 249.95853 15.66 Fluridone C19H14F3NO M + H 330.11003 310.1038 290.09757 13.62 Acephate C4H10NO3PS M + H 184.01918 142.99264 112.99968 5.61 Hexazinone C12H20N4O2 M + H 253.1659 171.08765 71.06037 10.59 Imazalil C14H14Cl2N2O M + H 297.0556 158.97628 255.00864 10.77 Imidacloprid C9H10ClN5O2 M + H 256.05958 209.05885 175.09793 9.3 Linuron C9H10Cl2N2O2 M + H 249.01921 159.97153 182.02414 14.09 Metribuzin C8H14N4OS M + H 215.09611 187.10119 131.03859 11.73 Myclobutanil C15H17ClN4 M + H 289.12145 70.03997 125.01525 14.21 Acetamiprid C10H11ClN4 M + H 223.0745 126.0105 98.9997 9.58 Norflurazon C12H9ClF3N3O M + H 304.0459 284.03967 160.03686 12.81 Propyzamide C12H11C12NO M + H 256.02905 189.9821 172.95555 14.68 Atrazine C8H14ClN5 M + H 216.10105 174.0541 96.05562 12.63 Simazine C7H12ClN5 M + H 202.0854 132.0323 104.001 11.35 Desethyl atrazine C6H10ClN5 M + H 188.06975 146.0228 104.001 9.37 Azoxystrobin C22H17N3O5 M + H 404.1241 344.10397 329.07949 14.2 Benoxacor C11H11Cl2NO2 M + H 260.02396 149.08352 120.04439 14.31 Other Scopolamine C17H21NO4 M + H 304.15433 156.10191 138.09134 7 substances Valnemulin C31H52N2O5S M + H 565.36697 263.1417 72.08078 11.15 Atropine C17H23NO3 M + H 290.17507 124.11208 93.06988 7.61 Gradient elution program in positive ion mode Time (min) A(%) B(%) 0 95 5 1.0 95 5 15 5 95 17 5 95 17.1 95 5 22.0 95 5 Gradient elution program in negative ion mode Time (min) A(%) B(%) 0 95 5 8 5 95 10 5 95 10.1 95 5 13 95 5 BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF EMBODIMENTS
1. Preparation of Standard Working Solutions
2. Pretreatment of Samples
3. Instrumental Analysis
Gradient elution program in positive ion mode Time (min) A(%) B(%) 0 90-95 10-5 1.0 90-95 10-5 15 5-10 95-10 17 5-10 95-90 17.1 95-90 5-10 22.0 95-90 5-10 Gradient elution program in negative ion mode Time (min) A(%) B(%) 0 90-95 10-5 8 5-10 95-90 10 5-10 95-90 10.1 95-90 5-10 13 95-90 5-10 4. Establishment of Spectrometry Database
Mass spectrometry library information Retention Molecular Addition Parent time Category Compound formula mode ion Fragment 1 Fragment 2 (min) Sulfonamides Sulfamethazine C12H14N4O2S M + H 279.09102 156.01138 124.08694 8.44 Sulformethoxine C12H14N4O4S M + H 311.08085 156.01138 140.04545 9.55 Sulfisoxazole C11H13N3O3S M + H 268.07504 156.0111 108.04462 9.83 Sulfaguanidine C7H10N4O2S M + H 215.05972 156.01138 108.04439 3.43 Sulfamerazine C11H12N4O2S M + H 265.07537 190.02809 156.01138 7.89 Sulfamethylthiadizaole C9H10N4O2S2 M + H 271.03179 156.01138 108.04439 8.31 Sulfamethoxazole C10H11N3O3S M + H 254.05939 156.01138 108.04439 9.57 Sulfamethoxydiazine C11H12N4O3S M + H 281.07029 108.04465 156.01115 8.53 Sulfamethoxypyridazine C11H12N4O3S M + H 281.07029 215.09274 156.01138 8.41 Sulfamonomethoxine C11H12N4O3S M + H 281.07029 215.09274 156.01138 8.9 Sulfamethazole C11H13N3O3S M + H 268.07504 156.0111 108.04462 8.03 Sulfapyridinee C11H11N3O2S M + H 250.06447 184.08692 156.01138 7.55 Sulfaquinoxaline C14H12N4O2S M + H 301.07537 156.01138 108.04439 10.39 Sulfathiazole C9H9N3O2S2 M + H 256.02089 156.01138 108.04439 7.28 Sulfisomidine C12H14N4O2S M + H 279.09102 124.08692 108.04439 6.57 Trimethoprim C14H18N4O3 M + H 291.14517 275.11387 261.09822 7.28 Diaveridine C13H16N4O2 M + H 261.1346 245.1033 123.06652 7 Phthalylsulfathiazole C17H13N3O5S2 M + H 404.03694 149.02313 256.02032 9.07 Sulfabenzamide C13H12N2O3S M + H 277.06414 156.01138 108.04439 10.32 Sulfacetamide C8H10N2O3S M + H 215.04849 173.03792 156.01138 6.73 Sulfachlorpyridazine C10H9ClN4O2S M + H 285.02075 156.01138 108.04439 9.25 Sulfachloropyrazine C10H9ClN4O2S M + H 285.02075 130.0166 156.01111 10.35 Sulfadiazine C10H10N4O2S M + H 251.05972 156.01138 108.04439 7.13 Sulfadimethoxine C12H14N4O4S M + H 311.08085 156.07675 108.04439 10.43 Fluoroquinolones Lomefloxacin C17H19F2N3O3 M + H 352.14672 308.1569 265.1147 7.58 Marbofloxacin C17H19FN4O4 M + H 363.14631 320.10411 72.08078 7.17 Nadifloxacin C19H21FN2O4 M + H 361.15581 343.14389 283.08725 10.76 Nalidixic acid C12H12N2O3 M + H 233.09207 205.06077 187.0502 11.43 Norfloxacin C16H18FN3O3 M + H 320.1405 276.15067 233.10847 7.24 Ofloxacin C18H20FN3O4 M + H 362.15106 318.16123 261.10338 7.31 Orbifloxacin C19H20F3N3O3 M + H 396.15295 352.16312 295.10527 7.89 Oxolinic acid C13H11NO5 M + H 262.071 244.06043 234.0397 9.95 Pipemidic acid C14H17N5O3 M + H 304.14042 290.16632 233.10847 6.75 Pefloxacin C17H20FN3O3 M + H 334.15615 217.10839 189.0779 7.35 Cinoxacin C12H10N2O5 M + H 263.06625 235.07133 217.06077 9.47 Sarafloxacin C20H17F2N3O3 M + H 386.13107 342.14125 299.09905 8.16 Sparfloxacin C19H22F2N4O3 M + H 393.17327 349.18344 292.12559 8.22 Ciprofloxacin C17H18FN3O3 M + H 332.1405 288.15067 245.10847 7.37 Danofloxacin C19H20FN3O3 M + H 358.15615 314.16632 283.12412 7.54 Difloxacin C21H19F2N3O3 M + H 400.14672 356.15689 299.09905 8.27 Enoxacin C15H17FN4O3 M + H 321.13575 277.14592 257.13969 7.13 Enrofloxacin C19H22FN3O3 M + H 360.1718 316.18197 245.10847 7.73 Fleroxacin C17H18F3N3O3 M + H 370.1373 326.14747 269.08962 7.36 Flumequine C14H12FNO3 M + H 262.0874 244.07683 220.04045 11.59 Gatifloxacin C19H22FN3O4 M + H 376.16671 332.17688 289.13468 7.97 Stimulants Cyproheptadine C21H21N M + H 288.17468 191.08553 96.08078 10.67 Fenoterol C17H21NO4 M + H 304.15433 286.14377 135.08044 6.57 Labetalol C19H24N2O3 M + H 329.18597 311.1754 294.14886 8.97 Penbutolol C18H29NO2 M + H 292.22711 236.16451 201.12739 11.08 Phenylethanolamine A C19H24N2O4 M + H 345.18088 327.17032 150.09134 10.03 Ractopamine C18H23NO3 M + H 302.17507 284.16451 164.10699 7.56 Salbutamol C13H21NO3 M + H 240.15942 148.07569 166.08625 5.75 Salmeterol C25H37NO4 M + H 416.27954 398.26897 380.2584 11.03 Terbutaline C12H19NO3 M + H 226.14377 170.08117 152.07061 5.71 Mabuterol C13H18ClF3N2O M + H 311.11325 237.03951 217.03343 9 Tulobuterol C12H18ClNO M + H 228.11497 172.05237 154.0418 8.25 Bambuterol C18H29N3O5 M + H 368.218 312.1554 294.14483 8.65 Brombuterol C12H18Br2N2O M + H 364.98586 290.9127 211.99436 8.71 Cimaterol C12H17N3O M + H 220.14444 202.13387 160.08692 5.93 Cimbuterol C13H19N3O M + H 234.16009 160.08656 143.06007 6.61 Clenbuterol C12H18Cl2N2O M + H 277.0869 259.07633 203.01373 8.31 Clenproperol C11H16Cl2N2O M + H 263.07125 245.06068 203.01373 7.85 Clonidine C9H9Cl2N3 M + H 230.02463 212.99808 / 6.77 Hormones Chlormadinone acetate C23H29ClO4 M + H 405.18271 309.1849 267.1743 15.67 17a-methyltestosterone C20H30O2 M + H 303.23186 285.22129 267.21073 13.63 Clobetasol propionate C25H32ClFO5 M + H 467.19951 263.143 147.0804 15.08 Clobetasone butyrate C26H32ClFO5 M + H 479.19951 279.138 343.1459 16.13 Corticosterone C21H30O4 M + H 347.22169 329.21112 311.20056 11.61 Hydrocortisone C21H30O5 M + H 363.2166 327.19547 309.18491 10.34 Cortisone acetate C23H30O6 M + H 403.21152 343.19038 163.11174 12.53 Cortisone C21H28O5 M + H 361.20095 163.1117 121.0648 10.45 Deflazacort C25H31NO6 M + H 442.22241 142.0499 312.1958 12.39 Dexamethasone C22H29FO5 M + H 393.20718 355.19039 337.17982 11.21 Diflorasone diacetate C26H32F2O7 M + H 495.21889 121.0648 317.1536 13.91 Epitestosterone C19H28O2 M + H 289.21621 97.0648 109.0648 13.93 Estradiol benzoate C25H28O3 M + H 377.21112 279.1743 321.1849 12.22 Fludrocortisone acetate C23H31FO6 M + H 423.21774 181.1023 239.143 12.22 Flumetasone pivalate C27H36F2O6 M + H 495.25527 57.0699 121.0648 15.25 Flumethasone C22H28F2O5 M + H 411.19776 235.1117 277.1587 11.2 Fluocinolone acetonide C24H30F2O6 M + H 453.20832 121.0648 337.1434 11.86 Fluorometholone C22H29FO4 M + H 377.21226 279.1743 135.0804 12.22 Flurandrenolide C24H33FO6 M + H 437.23339 361.1798 105.0699 12.01 Fluticasone propionate C25H31F3O5S M + H 501.19171 121.0648 275.143 15.08 Nandrolone propionate C21H30O3 M + H 331.22677 257.18999 239.17943 17.38 Halcinonide C24H32ClFO5 M + H 455.19951 121.0648 377.1514 14.98 Dihydrodiethylstilbestrol C18H22O2 M + H 271.16926 109.1117 253.1587 11.92 Medroxyprogesterone C24H34O4 M + H 387.25299 123.0804 327.2319 15.81 acetate Megestrol acetate C24H32O4 M + H 385.23734 267.1743 224.156 15.53 Melengestrol acetate C25H32O4 M + H 397.23734 279.1743 337.2162 15.82 Methandienone C20H28O2 M + H 301.21621 283.20564 149.13248 12.73 Methylprednisolone C22H30O5 M + H 375.2166 161.0961 135.0804 10.99 Norethindrone C20H26O2 M + H 299.20056 109.0648 281.19 13.12 Nandrolone propionate C18H26O2 M + H 275.20056 109.06499 239.17885 12.47 Levonorgestrel C21H28O2 M + H 313.21621 109.0648 245.19 14.23 Prednicarbate C27H36O8 M + H 489.24829 147.0804 289.1587 14.91 Prednisolone C21H28O5 M + H 361.20095 147.0804 171.0804 10.2 Prednisone C21H26O5 M + H 359.1853 341.17474 237.12739 10.27 Progesterone C21H30O2 M + H 315.23186 109.06479 97.06479 15.76 Dehydrotestosterone C19H26O2 M + H 287.20056 121.0647 135.1167 12.22 Testosterone C19H28O2 M + H 289.21621 109.06479 97.06479 13.03 Trenbolone C18H22O2 M + H 271.16926 253.15869 199.11174 11.92 Triamcinolone C24H31FO6 M + H 435.21774 397.201 339.1591 11.69 acetonide Beclomethasone C22H29ClO5 M + H 409.17763 391.1671 279.1743 11.45 Betamethasone C28H37FO7 M + H 505.25961 279.1741 319.1693 15.45 dipropionate Betamethasone C27H37FO6 M + H 477.26469 279.1743 147.0804 14.55 valerate Betamethasone C22H29FO5 M + H 393.20718 355.19039 279.17434 11.14 Nitroimidazoles Dimetridazole C5H7N3O2 M + H 142.0611 112.06311 95.06037 7.17 Ipronidazole C7H11N3O2 M + H 170.0924 140.09441 123.09167 10.33 Metronidazole C6H9N3O3 M + H 172.07167 128.04545 82.05255 6.39 Ornidazole C7H10ClN3O3 M + H 220.04835 128.04547 203.14304 8.77 Hydroxydimetridazole C5H7N3O3 M + H 158.05602 140.0455 110.0475 6.42 Ronidazole C6H8N4O4 M + H 201.06183 140.04545 110.04746 7.03 Secnidazole C7H11N3O3 M + H 186.08732 128.0455 111.0427 7.56 Tinidazole C8H13N3O4S M + H 248.06995 154.0611 110.08384 8.12 Antiviral Amantadine C10H17N M + H 152.14338 135.11683 107.08553 7.48 drugs Rimantadine; C12H21N M + H 180.17468 163.14778 81.0704 9 Lincosamides Clindamycin C18H33ClN2O5S M + H 425.18715 126.12773 / 8.69 Lincomycin C18H34N2O6S M + H 407.221 126.12773 359.21766 6.76 Amphenicols Chloramphenicol C11H12Cl2N2O5 M − H 321.00505 257.03346 194.04588 10.11 Florfenicol C12H14Cl2FNO4S M − H 355.99319 335.98696 185.02779 9.96 Thiamphenicol C12H15Cl2NO5S M − H 353.99752 290.02593 185.02779 8.27 β-Lactams Ceftiofur C19H17N5O7S3 M + H 524.03629 241.03899 210.02065 9.63 Flucloxacillin C19H17ClFN3O5S M + H 454.06342 160.04242 114.03742 12.5 Cloxacillin C19H18ClN3O5S M + H 436.07285 277.0374 206.0367 12.26 Nafcillin C21H22N2O5S M + H 415.13222 199.0754 171.0441 12.54 Piperacillin C23H27N5O7S M + H 518.1704 143.0815 115.0502 10.16 Penicillin G C16H18N2O4S M + H 335.106 217.06414 176.07061 10.77 Quinoxalines Quinocetone C18H14N2O3 M + H 307.10772 143.06018 273.10178 11.99 Antipyretics Phenacetin C10H13NO2 M + H 180.10191 110.0602 138.09109 10.11 and analgesics Salicylic acid C7H6O3 M − H 137.02442 93.0346 65.0397 3.74 Amidopyrine C13H17N3O M + H 232.14444 113.10738 98.08418 6.46 Antipyrine C11H12N2O M + H 189.10224 147.09135 161.10696 8.39 Dapsone C12H12N2O2S M + H 249.06922 156.01122 108.04431 9.03 Flunixin meglumine C14H11F3N2O2 M + H 297.08454 279.07397 264.0505 13.66 Acetaminophen C8H9NO2 M + H 152.07061 110.06004 / 6.25 Psychoactive Haloperidol C21H23ClFNO2 M + H 376.14741 358.13685 165.07102 10.3 drugs Imipramine C19H24N2 M + H 281.20123 86.0963 236.1434 10.71 Nitrazepam C15H11N3O3 M + H 282.08732 268.08383 207.09126 11.53 Oxazepam C15H11ClN2O2 M + H 287.05818 241.05203 269.04703 11.49 Pemoline C9H8N2O2 M + H 177.06585 106.0651 79.0542 7.74 Perphenazine C21H26ClN3OS M + H 404.15579 143.11763 171.14877 10.83 Promethazine C17H20N2S M + H 285.142 198.0372 86.09643 10.34 Propionylpromazine C20H24N2OS M + H 341.16821 86.0964 58.0651 10.95 hydrochloride Sulpiride C15H23N3O4S M + H 342.1482 214.0169 112.1121 6.21 Xylazine C12H16N2S M + H 221.1107 90.0372 164.0528 8.27 hydrochloride Acepromazin C19H22N2OS M + H 327.15256 254.06341 86.09643 10.2 Anisopirol C19H24FN3O M + H 330.19762 312.1865 121.076 7.44 Azaperone C19H22FN3O M + H 328.18197 165.07069 121.07603 8.04 Carbamazepine C15H12N2O M + H 237.10224 194.0964 220.0757 11.15 Chloropromazine C17H19ClN2S M + H 319.10302 86.09643 246.01379 11.25 Diazepam C16H13ClN2O M + H 285.07892 154.0415 193.0883 13.58 Diphenhydramine C17H21NO M + H 256.16959 167.0855 152.0621 10.03 hydrochloride Droperidol C22H22FN3O2 M + H 380.17688 165.07072 194.09726 9.35 Estazolam C16H11ClN4 M + H 295.0745 267.05524 205.0755 11.44 Macrolides Clarithromycin C38H69NO13 M + H 748.48417 158.11756 116.07061 11.04 Tiamulin C28H47NO4S M + H 494.32986 192.10528 119.01613 10.8 Erythromycin C37H67NO13 M + H 734.46852 158.11756 116.07061 9.92 Kitasamycin C40H67NO14 M + H 786.46343 558.32727 174.11247 11.25 Tilmicosin C46H80N2O13 M + H 869.57332 174.11247 88.07569 8.93 Tylosin C46H77NO17 M + H 916.52643 772.44778 174.11247 10.17 anthelmintics Mebendazole C16H13N3O3 M + H 296.10297 264.07675 105.03349 10.85 Mebendazole-amine C14H11N3O M + H 238.09749 105.03369 / 8.45 2-aminofluorobenzimidazole C14H10FN3O M + H 256.08807 123.0241 113.0397 8.74 Oxfendazole C15H13N3O3S M + H 316.07504 284.04882 223.0576 9.26 Oxibendazole C12H15N3O3 M + H 250.11862 218.0924 176.04545 9.33 Parbendazole C13H17N3O2 M + H 248.13935 216.1131 160.0505 10.65 5-hydroxythiabendazole C10H7N3OS M + H 218.03826 191.02736 / 6.44 Tiabendazole C10H7N3S M + H 202.04334 175.03245 131.06037 7.12 Albendazole C12H15N3O2S M + H 266.09577 234.06956 191.01478 10.99 Albendazole sulfone C12H15N3O4S M + H 298.0856 266.05939 224.01244 9.4 Albendazole sulfoxide C12H15N3O3S M + H 282.09069 240.04347 208.01752 8.08 Amino albendazole C10H13N3O2S M + H 240.08012 198.03317 165.05328 6.94 sulfone Benzimidazole C7H6N2 M + H 119.06037 92.0495 65.0386 4.8 Febantel C20H22N4O6S M + H 447.13328 383.08085 280.05391 14.78 Lobendazole C10H11N3O2 M + H 206.0924 160.0505 178.0609 7.59 toltrazuril sulfoxide C18H14F3N3O5S M − H 440.05335 371.05813 / 8.97 Clopidol C7H7Cl2NO M + H 191.99775 86.9996 101.01525 6.83 Dinitolmide C8H7N3O5 M − H 224.03129 181.02438 77.03858 9.91 Halofuginone C16H17BrClN3O3 M + H 414.02146 100.07598 120.08076 8.89 Levamisole C11H12N2S M + H 205.0794 178.0685 / 6.99 Pesticides Buprofezin C16H23N3OS M + H 306.16346 201.10561 116.05285 17.73 Carboxin C12H13NO2S M + H 236.07398 143.01613 93.0573 12.61 3-hydroxy carbofuran C12H15NO4 M + H 238.10738 163.07536 135.08044 9.09 Clothianidin C6H8ClN5O2S M + H 250.016 131.96692 113.0168 8.96 Cyromazine C6H10N6 M + H 167.10397 85.05087 125.08217 3.63 Diuron C9H10Cl2N2O M + H 233.02429 72.04439 159.97153 12.65 Ethopabate C12H15NO4 M + H 238.10738 206.08117 164.07061 10.29 Fipronil C12H4Cl2F6N4OS M − H 434.93143 329.95845 249.95853 15.66 Fluridone C19H14F3NO M + H 330.11003 310.1038 290.09757 13.62 Acephate C4H10NO3PS M + H 184.01918 142.99264 112.99968 5.61 Hexazinone C12H20N4O2 M + H 253.1659 171.08765 71.06037 10.59 Imazalil C14H14C12N2O M + H 297.0556 158.97628 255.00864 10.77 Imidacloprid C9H10ClN5O2 M + H 256.05958 209.05885 175.09793 9.3 Linuron C9H10Cl2N2O2 M + H 249.01921 159.97153 182.02414 14.09 Metribuzin C8H14N4OS M + H 215.09611 187.10119 131.03859 11.73 Myclobutanil C15H17ClN4 M + H 289.12145 70.03997 125.01525 14.21 Acetamiprid C10H11ClN4 M + H 223.0745 126.0105 98.9997 9.58 Norflurazon C12H9ClF3N3O M + H 304.0459 284.03967 160.03686 12.81 Propyzamide C12H11Cl2NO M + H 256.02905 189.9821 172.95555 14.68 Atrazine C8H14ClN5 M + H 216.10105 174.0541 96.05562 12.63 Simazine C7H12ClN5 M + H 202.0854 132.0323 104.001 11.35 Desethyl atrazine C6H10ClN5 M + H 188.06975 146.0228 104.001 9.37 Azoxystrobin C22H17N3O5 M + H 404.1241 344.10397 329.07949 14.2 Benoxacor C11H11Cl2NO2 M + H 260.02396 149.08352 120.04439 14.31 Other Scopolamine C17H21NO4 M + H 304.15433 156.10191 138.09134 7 substances Valnemulin C31H52N2O5S M + H 565.36697 263.1417 72.08078 11.15 Atropine C17H23NO3 M + H 290.17507 124.11208 93.06988 7.61 5. Sample Analysis
6. Compound Screening
7. Confirmation
8. Sensitivity Analysis
9. Method Validation
Recovery rate and precision of the method of the invention Average recovery rate (n = 5) Intra-batch RSD (n = 5) Detection Chicken Duck Cow's goat's Chicken Duck Cow's Goat's limit Compound egg egg Chicken Pork Mutton Beef milk milk egg egg Chicken Pork Mutton Beef milk milk μg/kg Sulfamethazine 107.33 105.30 117.10 100.00 118.19 106.94 97.81 115.59 5.71 12.84 16.03 12.63 16.77 12.37 9.29 6.03 5 Sulformethoxine 116.53 97.62 123.57 110.26 98.63 125.30 84.87 103.92 3.43 11.44 12.46 8.38 4.18 11.28 7.00 4.70 5 Sulfisoxazole 108.13 102.88 126.71 72.86 97.80 119.28 84.40 108.11 6.29 19.23 10.49 5.06 3.61 11.86 7.07 5.89 5 Sulfaguanidine 75.37 68.11 91.07 72.86 63.90 78.64 63.44 81.39 3.78 16.56 11.38 5.06 4.28 9.98 3.64 7.88 5 Sulfamerazine 109.57 105.62 106.40 106.05 125.02 109.46 96.08 109.08 3.81 12.37 13.09 11.80 10.03 11.75 14.49 6.65 5 Sulfamethylthiadizaole 107.15 89.54 121.58 117.05 92.51 109.88 75.09 98.90 4.59 18.76 13.89 8.33 2.62 11.99 12.23 9.12 5 Sulfamethoxazole 119.91 97.62 129.73 98.24 100.92 125.51 85.06 105.22 4.77 14.68 10.35 19.89 2.25 9.53 8.19 6.58 5 Sulfamethoxydiazine 108.36 97.01 110.06 98.24 115.37 103.30 114.77 113.79 2.72 16.20 10.84 19.89 14.99 11.90 11.70 7.86 5 Sulfamethoxypyridazine 108.36 97.01 110.88 110.38 115.37 103.30 128.93 113.79 2.72 16.20 19.33 13.28 14.99 11.90 22.57 9.40 5 Sulfamonomethoxine 116.02 100.53 127.83 81.00 97.78 121.97 81.79 99.84 4.66 13.33 10.24 1.87 3.08 10.42 7.51 16.23 5 Sulfamethazole 99.83 87.29 121.09 115.70 99.41 102.51 77.41 101.33 6.21 14.97 18.16 5.97 3.82 19.25 7.67 6.82 5 Sulfapyridinee 104.31 94.58 113.58 95.80 101.39 110.11 85.51 97.81 4.67 16.05 9.55 11.43 4.24 10.83 7.54 9.43 5 Sulfaquinoxaline 112.49 91.75 126.78 96.35 96.56 126.50 80.37 96.99 4.01 15.32 13.79 3.97 5.99 10.00 15.77 4.43 5 Sulfathiazole 104.11 96.65 117.51 95.17 101.83 104.97 81.59 97.61 5.05 16.86 12.34 1.98 3.22 13.87 7.35 8.25 5 Sulfisomidine 92.51 88.80 110.79 112.53 86.99 96.77 75.92 80.19 3.83 17.32 11.92 3.02 3.58 6.41 7.41 4.63 5 Trimethoprim 91.75 91.22 125.59 115.59 87.44 104.09 76.71 75.08 3.30 4.53 13.56 2.26 3.32 4.05 2.21 1.29 5 Diaveridine 85.49 84.27 107.60 111.96 87.36 79.32 77.69 74.87 3.72 5.52 7.36 2.75 2.48 4.88 2.82 1.40 5 Phthalylsulfathiazole 76.52 83.83 92.73 103.23 47.84 94.81 71.74 76.23 10.73 17.398 1.32 4.53 6.96 5.97 12.26 12.61 5 Sulfabenzamide 115.96 104.82 91.52 116.62 88.21 123.29 79.44 103.09 1.35 18.82 1.26 3.22 3.72 8.02 7.19 7.41 5 Sulfacetamide 101.31 101.84 82.67 115.32 95.93 114.60 85.38 105.49 4.83 13.11 4.57 4.54 2.24 7.02 6.98 3.94 5 Sulfachlorpyridazine 108.98 100.48 119.07 107.63 95.52 121.83 86.27 102.72 2.52 18.21 10.97 5.79 2.30 10.80 9.69 5.88 5 Sulfachloropyrazine 117.78 123.57 120.41 102.16 95.45 129.02 82.42 106.08 12.26 12.54 10.07 5.78 4.31 7.29 13.04 9.33 10 Sulfadiazine 99.39 94.53 110.89 89.61 98.44 111.94 82.01 97.77 5.54 14.84 11.93 3.30 3.60 11.08 7.74 4.55 5 Sulfadimethoxine 111.07 95.77 91.87 82.67 96.41 126.47 108.85 112.35 3.80 17.47 10.38 1.84 5.86 11.93 10.18 14.32 10 Lomefloxacin 81.03 80.48 107.61 117.37 67.89 99.85 53.42 68.06 2.47 6.25 10.08 6.24 3.71 5.29 22.46 10.09 5 Marbofloxacin 72.04 71.06 129.40 75.49 60.31 87.45 59.37 66.48 5.56 4.77 11.01 2.89 2.09 3.90 3.89 9.43 5 Nadifloxacin 109.80 103.73 88.75 122.99 97.52 117.22 82.32 103.66 2.19 12.67 1.54 2.59 4.58 7.78 16.72 5.42 5 Nalidixic acid 111.07 113.15 78.28 58.85 108.02 105.39 89.80 106.37 2.20 3.43 3.65 2.04 5.18 3.68 2.85 13.14 5 Norfloxacin 55.82 52.32 121.67 81.51 47.42 66.71 42.07 50.43 4.24 7.88 1.95 1.49 2.97 3.39 9.12 12.93 5 Ofloxacin 77.45 75.22 122.19 100.00 65.33 91.24 61.95 69.12 4.19 5.49 1.80 6.85 1.85 4.79 6.71 7.54 5 Orbifloxacin 97.83 100.86 55.97 81.00 88.97 116.12 85.47 85.89 4.36 7.13 1.71 3.17 1.34 4.69 4.23 4.33 1 Oxolinic acid 117.79 112.71 81.36 100.28 121.73 123.19 89.48 123.61 3.95 6.79 4.99 2.70 7.34 6.63 16.68 15.01 5 Pefloxacin 79.46 74.07 106.42 100.82 69.26 88.41 60.23 68.41 5.96 5.18 0.94 4.00 2.72 3.77 10.37 15.41 5 Cinoxacin 101.39 101.45 104.66 97.16 66.25 116.45 86.01 96.84 4.56 5.24 1.79 13.24 3.20 6.56 4.25 7.31 1 Sarafloxacin 103.64 96.36 106.00 66.16 96.00 117.72 70.59 79.18 2.51 8.15 3.36 2.49 2.54 6.55 4.59 11.31 5 Sparfloxacin 101.80 99.26 106.79 107.72 98.31 113.40 81.74 86.09 2.49 6.89 1.92 6.45 2.46 6.30 7.70 7.61 5 Ciprofloxacin 66.44 62.44 64.77 54.72 52.07 77.06 52.11 57.55 4.93 5.07 2.10 3.20 4.76 3.39 7.87 10.96 5 Danofloxacin 76.80 72.83 82.61 82.80 73.30 92.56 63.97 77.47 3.78 5.14 2.81 2.52 5.85 6.20 13.03 20.87 5 Difloxacin 106.01 98.81 119.85 115.01 109.12 123.33 89.41 96.66 2.48 7.11 3.00 3.51 1.94 5.84 9.02 15.08 5 Enoxacin 53.36 49.39 55.32 96.84 41.87 64.95 57.74 62.21 6.56 6.10 3.17 4.62 2.44 4.17 9.37 7.35 10 Enrofloxacin 98.69 92.73 98.13 84.62 89.95 110.79 71.81 81.96 3.46 5.73 1.98 1.66 2.87 4.99 4.87 16.67 5 Fleroxacin 82.81 82.61 84.37 88.46 63.08 97.19 67.11 74.19 5.42 5.44 3.28 7.26 2.18 5.44 5.62 7.05 5 Pipemidic acid 40.02 43.2 41.06 40.02 42.56 44.89 36.54 37.85 5.69 3.25 4.17 9.56 1.25 7.25 5.14 6.03 5 Flumequine 116.54 111.57 121.92 89.19 98.06 116.60 85.17 101.03 3.15 5.61 2.35 13.65 11.95 5.24 12.89 11.66 5 Gatifloxacin 96.49 93.81 96.60 70.51 83.92 111.58 77.44 78.67 4.61 3.67 2.62 2.08 1.52 3.16 14.34 9.29 1 Mebendazole 106.13 89.62 128.31 113.01 81.66 114.52 84.30 83.34 4.36 6.89 3.41 15.10 10.55 4.55 8.36 13.33 10 Mebendazole-amine 95.77 87.82 94.10 95.78 124.23 102.95 79.34 82.26 3.72 10.73 2.59 7.80 2.53 4.05 15.75 8.14 5 2-Aminofluorobenzimidazole 101.04 87.96 99.54 119.45 115.02 102.55 87.58 87.19 2.18 13.35 2.32 3.86 4.35 5.54 16.02 8.32 5 Oxfendazole 116.58 112.57 122.94 116.81 103.29 127.73 88.63 100.73 2.73 8.15 1.75 3.85 2.34 3.82 10.95 18.85 5 Oxibendazole 117.17 107.42 122.48 68.29 105.56 125.21 86.64 91.46 1.63 10.45 3.82 18.13 5.79 3.82 14.81 13.35 5 Parbendazole 111.03 86.08 126.67 117.72 48.78 127.27 96.08 80.95 1.82 11.65 6.97 1.78 13.29 3.91 8.32 4.23 10 5-Hydroxythiabendazole 118.25 98.67 104.65 100.02 99.40 105.76 87.83 87.72 6.70 10.65 2.75 4.01 7.84 5.08 7.29 6.76 1 Tiabendazole 113.43 105.37 114.61 76.46 106.20 49.93 85.37 83.94 3.36 8.21 2.35 1.88 2.95 4.64 16.74 8.36 1 Albendazole 80.66 87.93 125.10 100.31 54.60 114.37 80.74 79.71 15.44 13.00 7.51 1.32 15.69 7.35 10.13 8.62 10 Albendazole sulfone 118.53 112.57 124.94 119.77 106.23 110.67 89.06 100.30 1.64 5.67 1.64 15.13 1.81 4.56 3.95 8.60 5 Albendazole 111.30 105.67 120.21 63.20 102.28 121.43 101.04 95.93 4.93 3.74 1.52 6.09 3.47 4.05 17.02 11.97 5 sulfoxide Amino albendazole 95.20 92.47 91.10 84.41 95.20 96.86 82.79 86.35 4.12 6.65 1.09 2.83 1.33 4.12 1.44 3.66 5 sulfone Benzimidazole 93.54 87.61 90.77 86.90 91.85 97.27 40.21 83.30 3.02 7.72 1.42 4.19 1.67 3.97 4.55 2.59 5 Febantel 114.08 116.87 129.99 107.65 110.37 114.90 87.18 87.75 5.09 9.67 3.66 6.36 9.37 7.59 5.19 10.81 5 Lobendazole 113.88 110.35 123.01 106.47 112.90 129.35 98.20 93.69 1.64 5.86 6.77 5.49 2.75 4.23 8.63 16.17 1 Cyproheptadine 122.94 96.21 93.91 49.01 109.85 129.61 86.36 84.65 5.99 12.94 5.77 3.25 4.86 4.28 6.98 6.90 5 Fenoterol 97.17 89.27 112.96 119.51 98.65 91.59 74.73 87.81 9.31 7.78 3.50 5.04 4.22 5.89 7.71 15.34 1 Labetalol 102.47 100.02 122.06 65.71 119.16 117.35 89.16 86.51 3.28 9.09 3.02 1.89 3.94 4.21 6.79 7.38 1 Penbutolol 105.46 93.64 122.27 114.46 99.28 107.23 74.30 92.45 3.78 11.03 3.05 5.26 5.05 4.11 4.41 4.24 5 Phenylethanolamine A 113.04 106.47 109.98 118.25 122.10 126.13 90.23 87.01 4.02 9.73 1.37 12.55 2.41 3.91 7.04 6.12 5 Ractopamine 102.78 101.49 49.70 76.43 124.32 115.85 78.83 77.76 1.41 7.03 1.49 9.07 4.94 6.28 10.50 21.52 1 Salbutamol 48.61 43.56 50.98 84.95 45.18 55.02 48.61 53.92 4.94 4.95 5.15 2.08 2.01 3.90 3.26 4.61 5 Salmeterol 108.40 87.00 68.31 99.34 84.56 129.93 83.92 92.70 1.24 19.62 1.02 2.13 8.69 5.38 11.07 16.16 5 Terbutaline 63.33 59.54 117.89 107.38 56.94 73.44 58.44 69.08 3.86 5.97 2.26 5.44 2.05 3.22 3.41 3.70 1 Mabuterol 109.36 112.98 110.45 102.99 120.99 127.69 90.97 80.99 4.06 4.47 1.89 3.98 1.20 4.30 4.99 6.38 1 Tulobuterol 99.90 101.55 109.70 95.59 107.92 119.79 98.20 85.74 1.97 3.55 0.89 16.63 2.93 4.54 9.45 4.37 1 Bambuterol 105.10 108.19 113.98 78.27 109.55 127.02 40.87 83.69 2.22 3.61 1.50 3.88 6.37 5.31 8.18 8.55 5 Brombuterol 101.59 104.56 111.96 91.84 116.26 115.00 89.10 83.95 3.02 2.88 2.49 2.86 5.42 7.61 10.01 17.06 5 Cimaterol 78.22 75.90 87.06 95.31 74.56 91.23 69.93 76.73 3.47 9.41 2.78 7.62 2.40 3.73 2.83 3.33 5 Cimbuterol 93.45 88.62 109.10 99.61 90.91 106.20 82.09 83.69 2.89 7.62 2.96 4.63 4.17 3.89 4.89 4.61 1 Clenbuterol 104.64 104.33 109.44 105.20 115.41 114.84 102.37 90.63 2.67 6.52 1.34 18.25 1.55 2.66 4.80 9.55 1 Clenproperol 97.76 103.92 107.58 108.68 106.72 116.96 89.37 84.62 1.78 6.28 1.74 8.19 1.77 1.86 5.95 4.34 1 Clonidine 89.81 101.20 103.16 82.21 94.07 118.26 85.76 83.82 3.24 4.32 3.60 5.71 1.82 4.05 2.76 2.75 1 Chlormadinone 78.75 129.62 73.45 107.77 86.16 129.49 101.27 92.70 8.25 19.87 5.71 6.50 6.89 3.36 9.61 8.26 50 acetate 17a-Methyltestosterone 102.28 109.75 122.00 116.22 102.18 129.58 96.14 90.16 4.31 9.17 2.34 4.39 2.98 4.35 7.58 12.18 10 Clobetasol 102.11 126.78 128.38 101.60 119.61 118.46 95.81 90.12 8.07 6.07 4.68 7.72 10.42 4.99 5.24 9.15 10 propionate Clobetasone butyrate 89.10 127.99 96.81 121.04 117.08 119.22 119.05 130.00 14.71 8.62 8.15 3.48 12.46 5.94 9.95 8.99 50 Corticosterone 104.54 112.30 121.64 104.67 104.70 126.14 88.66 93.38 9.70 4.44 3.11 3.67 1.91 4.75 11.10 8.69 5 Hydrocortisone 102.37 115.69 116.91 103.92 97.81 118.04 87.45 97.98 3.90 8.46 1.90 5.38 1.23 4.14 4.57 5.35 5 Cortisone acetate 106.52 122.12 123.53 123.50 41.24 86.49 94.96 85.53 7.01 8.77 2.52 5.95 10.12 7.37 5.86 7.72 50 Cortisone 107.17 101.68 123.26 108.89 102.05 121.62 107.72 97.72 9.45 9.75 1.30 9.83 2.77 4.34 3.31 2.51 10 Deflazacort 100.00 112.58 117.92 116.69 42.34 104.06 88.44 92.51 2.41 3.16 2.51 2.29 4.18 6.43 3.20 3.48 5 Dexamethasone 99.67 113.52 121.99 110.12 108.49 124.95 87.95 90.08 3.37 6.00 1.53 4.61 1.77 3.43 9.03 12.72 5 Diflorasone 106.11 117.12 126.18 111.55 89.49 126.41 102.46 92.73 6.68 14.10 3.44 15.34 8.07 5.33 3.79 9.87 10 diacetate Epitestosterone 95.30 115.25 123.78 113.47 111.03 128.48 96.72 87.38 6.05 6.33 1.82 5.20 1.88 3.62 8.39 10.37 10 Estradiol benzoate 103.64 85.86 122.19 120.55 103.86 127.50 87.18 100.73 3.59 17.18 3.42 11.34 7.91 4.54 14.30 12.78 5 Fludrocortisone 101.21 118.61 121.13 108.27 43.42 62.39 89.50 87.04 5.45 12.24 2.91 8.31 3.28 11.22 13.68 17.81 50 acetate Flumetasone 82.19 123.14 125.12 109.72 80.42 101.30 65.75 73.52 6.85 15.28 4.27 6.17 9.43 6.44 8.47 9.46 50 pivalate Flumethasone 101.50 77.98 124.79 121.29 127.98 126.82 90.67 91.00 5.58 9.21 1.87 8.14 3.26 4.78 7.56 14.22 5 Fluocinolone 111.14 108.57 123.59 125.87 129.61 128.75 88.87 92.62 5.28 8.97 3.38 4.63 3.64 4.14 8.18 12.59 5 acetonide Fluorometholone 103.64 106.37 121.97 110.86 103.82 127.50 87.21 100.34 3.59 12.42 3.61 9.60 7.85 4.54 14.32 11.56 5 Flurandrenolide 112.40 118.69 124.71 124.41 109.74 123.83 98.33 89.96 4.90 12.30 2.38 4.22 4.05 5.24 4.67 3.56 50 Fluticasone 105.95 68.87 128.81 90.55 82.62 122.51 85.04 89.19 16.51 5.94 5.04 6.68 7.35 5.64 6.31 6.15 50 propionate Nandrolone 91.46 106.35 122.04 86.40 43.36 121.22 84.24 82.22 6.36 10.36 8.41 6.89 3.91 4.47 8.61 7.49 50 propionate Halcinonide 86.90 103.46 124.03 93.66 112.06 121.88 87.18 85.26 13.56 13.18 6.71 4.72 11.61 3.35 9.54 7.89 50 Dihydrodiethylstilbestrol 113.62 115.08 115.87 103.69 103.70 125.15 86.91 78.62 13.23 6.38 3.80 7.83 4.25 4.34 11.90 17.91 1 Medroxyprogesterone 96.83 111.83 126.78 106.63 89.54 112.74 99.20 97.86 17.30 6.93 6.26 9.69 7.53 4.69 17.82 12.09 10 acetate Megestrol acetate 77.98 112.02 129.20 112.16 96.32 112.30 87.06 86.59 14.53 12.51 5.92 13.58 7.71 4.51 18.55 9.82 5 Melengestrol acetate 78.71 114.69 116.75 103.58 87.61 129.43 92.61 98.40 7.75 10.02 6.99 13.50 8.03 4.38 3.82 7.62 10 Methandienone 122.40 94.59 120.52 110.69 110.81 120.69 85.07 84.03 10.01 6.83 3.38 3.83 2.37 4.19 6.86 6.72 5 Methylprednisolone 86.06 121.73 115.98 109.74 102.62 121.62 90.30 95.71 4.30 11.72 1.67 3.19 1.57 3.60 5.55 9.52 5 Norethindrone 93.24 109.40 113.00 106.57 103.29 120.79 86.39 87.89 7.26 5.69 9.82 6.38 2.42 4.67 8.82 12.02 5 Nandrolone 101.54 106.17 120.39 111.03 111.57 121.59 83.98 86.25 3.06 7.87 5.42 5.85 4.12 4.84 9.31 10.96 5 propionate Levonorgestrel 94.79 115.46 119.71 107.92 108.16 113.16 95.42 87.40 6.10 8.25 5.41 11.23 3.48 4.98 18.04 9.21 10 Prednicarbate 92.11 57.61 114.54 110.81 99.27 107.83 99.23 96.62 3.08 7.31 3.72 11.55 10.05 6.24 17.00 11.52 10 Prednisolone 94.97 74.25 111.69 108.46 104.79 121.68 89.97 92.15 2.46 6.35 4.02 3.92 1.83 2.95 3.46 6.51 5 Prednisone 103.02 106.59 113.09 114.25 104.50 128.34 93.24 92.60 4.20 12.79 2.26 4.15 2.41 4.83 2.79 3.48 5 Progesterone 98.45 106.35 125.68 98.70 76.21 123.36 87.03 86.47 3.54 10.36 4.87 4.19 9.82 3.79 4.12 9.26 10 Dehydrotestosterone 99.53 125.20 120.52 110.97 103.13 110.17 88.64 93.53 4.16 6.69 2.68 3.91 2.19 4.52 6.76 6.34 1 Testosterone 98.74 113.10 122.23 103.35 105.15 105.10 84.63 88.13 8.85 7.57 3.81 5.65 3.01 5.44 11.71 14.76 5 Trenbolone 95.74 105.58 115.87 103.69 103.70 125.15 86.91 78.62 6.33 15.90 3.80 7.83 4.25 4.34 11.90 17.91 1 Triamcinolone 98.53 122.38 123.73 112.84 108.82 127.52 94.06 97.37 3.41 5.00 2.14 3.46 3.47 2.39 18.13 17.03 5 acetonide Beclomethasone 100.20 113.52 115.32 105.53 108.87 110.43 88.82 91.11 3.30 6.00 3.51 15.16 3.48 3.55 12.30 17.79 5 Betamethasone 102.16 98.48 112.97 101.51 81.40 120.46 90.60 91.41 4.58 14.32 8.38 3.21 16.05 2.83 8.47 15.25 5 dipropionate Betamethasone 98.38 103.88 126.08 101.14 99.28 128.17 88.80 82.19 7.11 13.07 7.24 8.23 5.45 5.84 6.08 15.73 5 valerate Betamethasone 99.67 103.54 117.89 110.12 108.49 124.95 87.95 90.08 3.37 6.87 2.83 4.61 1.77 3.43 9.03 12.72 5 Dimetridazole 106.15 118.97 112.51 120.10 111.14 98.37 100.23 67.27 5.83 2.18 4.46 7.45 6.03 18.01 8.28 12.42 5 Ipronidazole 107.61 98.10 128.72 85.37 123.02 96.74 100.26 60.49 6.12 4.66 3.19 7.23 9.18 17.20 8.19 17.14 5 Metronidazole 104.23 116.98 102.72 90.36 91.45 114.69 71.08 85.94 3.15 2.98 1.17 5.82 3.89 3.91 13.97 3.93 5 Omidazole 115.05 108.52 124.84 93.49 101.85 121.46 92.13 92.53 4.10 5.59 1.69 1.88 1.57 4.35 5.39 3.98 5 Hydroxydimetridazole 107.73 114.80 99.40 114.00 86.51 111.99 82.02 83.11 5.23 3.79 2.77 2.32 2.11 3.18 2.98 3.73 5 Ronidazole 105.28 67.72 109.14 91.17 100.58 118.46 85.57 90.68 2.75 4.25 3.78 3.75 3.07 2.84 6.61 5.23 5 Secnidazole 109.61 85.96 113.83 106.63 99.88 124.34 74.49 90.94 3.64 5.11 2.50 1.84 1.63 4.69 8.17 3.89 5 Tinidazole 113.20 54.52 113.42 106.33 105.73 127.16 92.86 92.87 2.23 9.34 4.93 2.21 4.00 3.49 2.67 6.02 5 Amantadine 87.01 98.69 73.23 70.07 76.37 81.25 83.61 73.62 2.39 3.36 1.95 4.78 2.23 2.50 3.76 2.01 1 Rimantadine 97.01 93.87 98.35 88.85 117.24 102.96 84.80 79.93 8.97 8.89 2.06 1.57 2.08 3.32 4.57 2.70 1 Clindamycin 67.37 94.19 75.04 68.95 89.71 82.51 76.88 75.66 5.42 10.41 2.81 6.16 4.44 6.72 7.81 6.87 10 Lincomycin 79.30 97.95 108.93 98.33 102.12 124.20 85.89 93.37 6.80 15.56 2.34 3.54 2.28 3.51 5.75 7.61 10 Chloramphenicol 105.07 98.69 120.22 125.63 94.37 127.62 100.56 102.31 2.86 3.36 1.71 14.95 2.52 4.79 5.69 1.91 1 Florfenicol 107.91 101.24 125.32 128.64 98.51 122.57 100.68 104.23 2.74 12.11 1.90 14.68 4.43 5.49 4.78 1.77 1 Thiamphenicol 104.32 98.76 118.80 109.24 96.44 113.45 98.65 102.52 2.99 9.76 8.75 17.50 3.64 3.13 7.36 2.24 1 Ceftiofur 76.55 106.17 81.99 77.33 48.18 94.09 88.19 84.90 4.69 9.41 3.97 4.30 6.19 3.70 8.96 7.26 10 Flucioxacillin 93.84 49.40 119.42 95.30 101.70 110.39 115.87 119.21 12.87 4.08 15.40 18.62 10.67 15.87 10.25 8.25 50 Cloxacillin 83.85 86.49 125.40 120.41 101.21 123.95 120.38 94.88 11.63 3.52 2.48 5.97 15.88 15.79 17.40 5.33 50 Nafcillin 99.68 121.79 128.20 117.70 114.65 116.76 129.63 104.31 5.01 5.15 5.15 6.26 19.08 8.93 18.41 19.99 50 Piperacillin 71.43 81.86 91.00 77.78 60.61 94.75 117.89 72.11 7.98 8.41 4.01 3.59 5.69 12.68 11.72 14.31 10 Penicillin G 53.89 73.48 100.46 78.30 63.58 90.22 120.26 108.04 16.44 4.68 6.70 7.10 11.79 13.18 12.04 9.51 10 Quinocetone 115.85 101.56 78.22 73.76 100.85 88.33 114.24 104.38 19.66 5.76 6.40 5.76 16.03 5.18 3.25 7.30 5 Phenacetin 114.22 89.19 120.73 113.03 104.63 129.27 89.58 86.65 5.03 9.84 2.31 2.11 2.37 4.38 8.06 11.04 5 Salicylic acid 62.71 73.48 62.45 72.77 54.93 88.88 50.93 54.51 6.19 4.68 2.06 16.06 7.40 6.42 7.56 4.51 10 Amidopyrine 83.29 92.69 105.75 126.45 89.33 110.86 72.24 125.36 10.21 16.70 2.48 9.58 19.60 10.22 4.21 5.52 10 Antipyrine 98.70 101.41 112.06 82.41 100.09 120.87 89.90 86.43 2.25 8.08 2.69 7.83 3.65 4.97 12.68 13.31 1 Dapsone 108.33 102.99 108.10 125.99 103.05 125.01 53.86 125.03 3.40 12.06 3.06 7.35 4.41 16.69 3.68 20.36 1 Flunixin meglumine 69.12 97.91 102.21 95.29 92.31 119.65 85.39 77.41 8.95 10.68 2.92 7.41 6.23 3.82 7.47 15.00 10 Acetaminophen 78.14 63.29 100.93 113.26 86.95 107.26 99.67 93.69 5.97 10.02 3.02 4.88 5.44 14.77 4.29 4.50 10 Haloperidol 128.37 96.68 122.37 105.46 129.29 123.64 94.14 84.93 5.95 12.11 3.01 4.06 4.73 4.80 7.84 10.60 5 Imipramine 123.20 98.01 122.38 110.75 128.27 129.29 94.11 87.12 4.06 17.24 3.12 3.27 4.78 6.20 5.18 5.67 1 Nitrazepam 122.31 77.98 120.04 109.52 106.64 127.43 89.72 84.32 3.49 6.84 1.73 3.14 4.19 4.26 6.88 14.93 5 Oxazepam 126.69 96.59 111.83 103.54 96.54 125.59 92.64 86.74 8.30 4.34 1.25 3.80 3.55 4.19 6.37 7.98 1 Pemoline 100.27 98.50 101.59 98.66 93.22 112.07 90.27 91.51 8.98 12.64 1.17 1.32 2.23 3.17 7.73 7.51 1 Perphenazine 123.23 107.94 123.31 95.22 40.77 108.19 71.73 68.97 10.84 7.23 15.11 14.95 16.37 5.39 11.82 9.60 5 Promethazine 127.29 115.21 118.33 125.59 81.62 111.89 85.47 85.00 7.92 4.55 3.80 8.58 12.12 12.06 4.67 5.31 1 Propionylpromazine 126.01 110.05 123.25 119.12 74.20 118.90 85.16 82.00 9.00 3.10 5.82 8.83 13.65 10.94 9.12 10.69 5 hydrochloride Sulpiride 74.14 83.94 82.60 78.33 71.51 94.84 72.40 66.27 6.34 7.04 1.95 2.58 8.39 5.74 3.19 4.54 5 Xylazine 88.54 110.65 110.00 104.87 107.24 119.56 87.93 86.58 6.12 6.20 2.13 5.92 3.51 5.67 5.12 3.19 1 hydrochloride Acepromazin 129.97 117.96 117.39 113.32 97.75 114.26 88.10 84.10 9.83 6.09 3.50 8.23 9.51 10.94 4.85 5.44 1 Anisopirol 107.69 109.21 115.45 114.37 115.76 124.77 91.04 90.25 3.01 9.58 1.43 1.86 4.49 2.87 4.78 2.62 1 Azaperone 115.67 113.48 114.64 120.76 95.86 128.45 92.21 88.55 4.04 2.57 2.53 7.75 9.96 4.14 6.09 13.20 1 Carbamazepine 105.06 93.61 117.25 110.90 99.85 124.52 97.11 94.91 3.72 6.60 2.06 1.66 4.88 5.03 3.53 2.92 1 Chloropromazine 107.39 116.52 122.19 126.84 58.14 118.44 78.54 84.75 3.72 5.87 7.22 15.57 16.76 11.96 13.89 15.28 5 Diazepam 49.43 111.03 119.16 92.36 104.65 128.39 86.19 77.60 7.52 5.15 1.89 4.09 4.93 4.91 6.22 7.04 1 Diphenhydramine 117.74 104.51 118.48 98.96 99.93 125.42 94.06 93.05 5.58 10.28 2.34 4.12 5.44 3.99 3.89 3.68 1 hydrochloride Droperidol 110.74 94.32 122.66 108.16 90.64 117.54 91.37 98.22 6.29 14.09 2.58 2.67 6.20 3.23 10.67 13.02 1 Estazolam 122.10 111.96 120.23 114.43 111.84 117.32 90.90 98.08 13.01 7.81 1.77 3.03 7.92 5.05 5.46 4.31 1 Clarithromycin 106.86 112.43 129.64 118.34 127.93 115.09 87.99 91.35 3.47 3.84 3.05 1.72 2.64 4.68 2.03 3.99 5 Tiamulin 107.33 112.22 125.96 116.60 102.73 111.81 100.79 97.17 1.92 3.88 2.95 1.97 2.44 5.00 4.85 5.27 5 Erythromycin 113.64 114.05 92.70 65.03 92.71 116.61 112.98 128.89 3.31 7.07 14.02 10.02 17.18 14.09 10.29 10.01 10 Kitasamycin 84.71 106.40 99.79 124.59 55.11 71.88 114.25 118.65 13.51 19.16 6.05 17.39 18.86 13.45 9.27 11.89 50 Tilmicosin 97.96 103.30 124.25 111.09 120.80 118.81 115.84 91.30 5.41 13.35 6.08 1.97 3.29 3.78 4.72 6.38 50 Tylosin 92.93 71.74 95.77 105.44 63.99 76.42 118.55 111.52 2.12 7.85 3.41 17.34 11.20 17.25 15.87 12.86 10 Toltrazuril sulfoxide 109.25 114.05 127.16 106.61 100.72 121.17 104.56 99.62 4.92 7.07 4.17 11.17 3.21 2.47 8.65 11.35 10 Clopidol 90.14 95.62 97.19 93.05 86.74 109.32 92.33 84.57 5.05 7.08 1.14 1.66 2.17 3.94 3.20 1.66 1 Dinitolmide 103.31 71.74 116.97 123.69 97.06 124.53 104.41 96.40 4.26 7.85 1.35 12.60 2.94 4.29 4.86 1.07 10 Halofuginone 70.44 77.60 88.60 90.78 103.34 100.83 85.63 81.77 3.75 10.19 4.02 7.05 5.96 6.44 7.38 7.99 10 Levamisole 92.81 106.75 103.76 97.12 96.85 116.27 82.84 85.35 5.75 9.77 1.32 2.28 1.74 4.22 2.12 2.01 5 Buprofezin 69.15 111.58 117.74 104.67 69.20 115.68 95.71 89.23 16.80 16.74 4.57 3.66 8.66 2.40 6.12 9.16 5 Carboxin 128.42 114.79 120.52 126.03 101.03 115.15 94.17 76.45 11.56 5.57 2.41 2.90 14.33 15.95 5.93 7.06 10 3-Hydroxycarbofuran 102.23 121.30 121.18 107.42 58.32 129.54 104.91 96.44 2.27 8.90 4.14 5.09 15.73 2.95 5.15 3.10 10 Clothianidin 100.67 102.14 122.77 114.71 97.64 113.95 106.26 96.72 5.84 11.58 3.62 2.90 1.99 3.89 4.40 3.64 10 Cyromazine 50.87 47.96 46.67 75.40 46.51 45.03 59.53 57.28 8.00 3.11 2.66 1.98 3.06 4.19 1.06 2.15 10 Diuron 123.12 114.05 123.49 113.10 101.83 128.10 95.31 85.99 7.51 4.26 2.53 9.75 1.60 3.22 17.47 9.86 10 Ethopabate 97.07 113.89 120.67 111.75 107.17 123.01 90.18 93.62 7.13 5.21 2.94 4.68 13.92 3.14 4.92 6.49 5 Fipronil 107.91 60.46 119.15 116.44 89.89 119.16 102.74 104.60 5.21 4.41 17.92 16.36 6.10 8.26 6.45 10.46 10 Fluridone 98.62 107.17 124.11 108.42 70.59 122.24 101.86 93.49 3.12 8.43 2.83 7.88 3.03 4.16 15.13 19.52 10 Acephate 69.28 113.70 82.98 82.76 105.45 79.01 81.65 75.20 6.80 4.00 4.10 2.65 2.06 4.24 2.89 4.21 10 Hexazinone 101.50 114.90 122.89 121.84 121.35 118.91 100.72 97.85 2.31 5.52 2.06 4.32 2.94 1.13 2.23 3.12 10 Imazalil 95.33 119.23 127.32 128.45 104.38 118.91 102.39 90.93 2.66 17.27 3.59 2.77 1.03 2.54 3.22 8.71 10 Imidacloprid 98.65 78.83 122.85 118.74 93.96 121.01 107.34 95.91 4.29 9.83 2.19 3.54 2.30 2.02 3.62 4.54 10 Linuron 100.08 113.24 123.08 111.60 103.58 127.59 90.49 74.90 3.67 9.03 3.77 12.30 1.57 3.79 11.24 8.26 10 Metribuzin 102.48 92.83 126.03 115.57 97.33 124.88 93.37 85.85 2.82 9.89 2.71 2.60 5.81 2.14 14.10 12.87 5 Myclobutanil, 100.72 109.80 126.18 121.11 101.49 126.01 91.26 97.30 2.87 3.85 2.42 4.26 3.58 2.15 7.16 11.01 5 Acetamiprid 97.83 114.85 123.57 84.65 107.39 123.45 98.57 94.28 3.46 4.71 1.82 8.73 3.65 2.31 1.80 4.06 10 Norflurazon 98.95 79.67 127.52 117.07 68.71 125.56 103.14 96.69 5.56 9.43 2.12 3.08 10.19 1.81 7.91 8.44 10 Propyzamide 98.87 119.15 127.33 113.55 99.07 124.81 96.22 71.15 4.55 5.75 1.84 8.58 3.42 3.32 16.02 19.06 50 Atrazine 88.80 95.51 129.21 116.81 102.43 128.46 89.12 77.37 8.02 15.36 1.41 2.65 1.89 1.53 3.01 8.89 5 Simazine 54.74 61.79 122.97 120.34 102.81 126.71 90.33 76.06 10.80 7.79 2.61 2.99 1.36 2.08 4.15 11.37 5 Desethyl atrazine 99.77 67.22 125.26 127.57 121.25 120.78 101.41 87.52 2.82 6.60 1.96 2.45 6.06 2.55 3.06 2.68 10 Azoxystrobin 103.10 83.63 129.88 118.44 112.11 123.62 91.49 90.10 2.01 16.42 1.34 3.59 9.16 8.92 5.70 8.55 5 Benoxacor 105.02 94.24 111.70 105.70 98.43 122.59 92.47 46.93 5.01 5.43 8.08 11.16 12.85 7.86 9.40 19.82 50 Scopolamine 99.57 76.10 120.33 90.26 70.95 76.19 66.52 69.30 1.78 7.82 5.62 1.60 3.18 1.75 2.31 2.36 5 Valnemulin 101.89 83.79 116.39 122.77 96.23 119.38 83.76 92.29 2.17 6.10 2.45 4.94 3.76 4.60 9.08 14.38 5 Atropine 99.74 76.60 120.95 89.87 94.80 87.52 75.00 73.48 1.93 9.18 1.97 2.18 7.29 1.55 6.35 20.00 1 Mass spectrometry library information Retention Molecular Addition Parent time Category Compound formula mode ion Fragment 1 Fragment 2 (min) Sulfonamides Sulfamethazine C12H14N4O2S M + H 279.09102 156.01138 124.08694 8.44 Sulformethoxine C12H14N4O4S M + H 311.08085 156.01138 140.04545 9.55 Sulfisoxazole C11H13N3O3S M + H 268.07504 156.0111 108.04462 9.83 Sulfaguanidine C7H10N4O2S M + H 215.05972 156.01138 108.04439 3.43 Sulfamerazine C11H12N4O2S M + H 265.07537 190.02809 156.01138 7.89 Sulfamethylthiadizaole C9H10N4O2S2 M + H 271.03179 156.01138 108.04439 8.31 Sulfamethoxazole C10H11N3O3S M + H 254.05939 156.01138 108.04439 9.57 Sulfamethoxydiazine C11H12N4O3S M + H 281.07029 108.04465 156.01115 8.53 Sulfamethoxypyridazine C11H12N4O3S M + H 281.07029 215.09274 156.01138 8.41 Sulfamonomethoxine C11H12N4O3S M + H 281.07029 215.09274 156.01138 8.9 Sulfamethazole C11H13N3O3S M + H 268.07504 156.0111 108.04462 8.03 Sulfapyridinee C11H11N3O2S M + H 250.06447 184.08692 156.01138 7.55 Sulfaquinoxaline C14H12N4O2S M + H 301.07537 156.01138 108.04439 10.39 Sulfathiazole C9H9N3O2S2 M + H 256.02089 156.01138 108.04439 7.28 Sulfisomidine C12H14N4O2S M + H 279.09102 124.08692 108.04439 6.57 Trimethoprim C14H1SN4O3 M + H 291.14517 275.11387 261.09822 7.28 Diaveridine C13H16N4O2 M + H 261.1346 245.1033 123.06652 7 Phthalylsulfathiazole C17H13N3O5S2 M + H 404.03694 149.02313 256.02032 9.07 Sulfabenzamide C13H12N2O3S M + H 277.06414 156.01138 108.04439 10.32 Sulfacetamide C8H10N2O3S M + H 215.04849 173.03792 156.01138 6.73 Sulfachlorpyridazine C10H9ClN4O2S M + H 285.02075 156.01138 108.04439 9.25 Sulfachloropyrazine C10H9ClN4O2S M + H 285.02075 130.0166 156.01111 10.35 Sulfadiazine C10H10N4O2S M + H 251.05972 156.01138 108.04439 7.13 Sulfadimethoxine C12H14N4O4S M + H 311.08085 156.07675 108.04439 10.43 Fluoroquinolones Lomefloxacin C17H19F2N3O3 M + H 352.14672 308.1569 265.1147 7.58 Marbofloxacin C17H19FN4O4 M + H 363.14631 320.10411 72.08078 7.17 Nadifloxacin C19H21FN2O4 M + H 361.15581 343.14389 283.08725 10.76 Nalidixic acid C12H12N2O3 M + H 233.09207 205.06077 187.0502 11.43 Norfloxacin C16H18FN3O3 M + H 320.1405 276.15067 233.10847 7.24 Ofloxacin C18H20FN3O4 M + H 362.15106 318.16123 261.10338 7.31 Orbifloxacin C19H20F3N3O3 M + H 396.15295 352.16312 295.10527 7.89 Oxolinic acid C13H11NO5 M + H 262.071 244.06043 234.0397 9.95 Pipemidic acid C14H17N5O3 M + H 304.14042 290.16632 233.10847 6.75 Pefloxacin C17H20FN3O3 M + H 334.15615 217.10839 189.0779 7.35 Cinoxacin C12H10N2O5 M + H 263.06625 235.07133 217.06077 9.47 Sarafloxacin C20H17F2N3O3 M + H 386.13107 342.14125 299.09905 8.16 Sparfloxacin C19H22F2N4O3 M + H 393.17327 349.18344 292.12559 8.22 Ciprofloxacin C17H18FN3O3 M + H 332.1405 288.15067 245.10847 7.37 Danofloxacin C19H20FN3O3 M + H 358.15615 314.16632 283.12412 7.54 Difloxacin C21H19F2N3O3 M + H 400.14672 356.15689 299.09905 8.27 Enoxacin C15H17FN4O3 M + H 321.13575 277.14592 257.13969 7.13 Enrofloxacin C19H22FN3O3 M + H 360.1718 316.18197 245.10847 7.73 Fleroxacin C17H18F3N3O3 M + H 370.1373 326.14747 269.08962 7.36 Flumequine C14H12FNO3 M + H 262.0874 244.07683 220.04045 11.59 Gatifloxacin C19H22FN3O4 M + H 376.16671 332.17688 289.13468 7.97 Stimulants Cyproheptadine C21H21N M + H 288.17468 191.08553 96.08078 10.67 Fenoterol C17H21NO4 M + H 304.15433 286.14377 135.08044 6.57 Labetalol C19H24N2O3 M + H 329.18597 311.1754 294.14886 8.97 Penbutolol C18H29NO2 M + H 292.22711 236.16451 201.12739 11.08 Phenylethanolamine A C19H24N2O4 M + H 345.18088 327.17032 150.09134 10.03 Ractopamine C18H23NO3 M + H 302.17507 284.16451 164.10699 7.56 Salbutamol C13H21NO3 M + H 240.15942 148.07569 166.08625 5.75 Salmeterol C25H37NO4 M + H 416.27954 398.26897 380.2584 11.03 Terbutaline C12H19NO3 M + H 226.14377 170.08117 152.07061 5.71 Mabuterol C13H18ClF3N2O M + H 311.11325 237.03951 217.03343 9 Tulobuterol C12H18ClNO M + H 228.11497 172.05237 154.0418 8.25 Bambuterol C18H29N3O5 M + H 368.218 312.1554 294.14483 8.65 Brombuterol C12H18Br2N2O M + H 364.98586 290.9127 211.99436 8.71 Cimaterol C12H17N3O M + H 220.14444 202.13387 160.08692 5.93 Cimbuterol C13H19N3O M + H 234.16009 160.08656 143.06007 6.61 Clenbuterol C12H18Cl2N2O M + H 277.0869 259.07633 203.01373 8.31 Clenproperol C11H16Cl2N2O M + H 263.07125 245.06068 203.01373 7.85 Clonidine C9H9Cl2N3 M + H 230.02463 212.99808 / 6.77 Hormones Chlormadinone acetate C23H29ClO4 M + H 405.18271 309.1849 267.1743 15.67 17a-methyltestosterone C20H30O2 M + H 303.23186 285.22129 267.21073 13.63 Clobetasol propionate C25H32ClFO5 M + H 467.19951 263.143 147.0804 15.08 Clobetasone butyrate C26H32ClFO5 M + H 479.19951 279.138 343.1459 16.13 Corticosterone C21H30O4 M + H 347.22169 329.21112 311.20056 11.61 Hydrocortisone C21H30O5 M + H 363.2166 327.19547 309.18491 10.34 Cortisone acetate C23H30O6 M + H 403.21152 343.19038 163.11174 12.53 Cortisone C21H28O5 M + H 361.20095 163.1117 121.0648 10.45 Deflazacort C25H31NO6 M + H 442.22241 142.0499 312.1958 12.39 Dexamethasone C22H29FO5 M + H 393.20718 355.19039 337.17982 11.21 Diflorasone diacetate C26H32F2O7 M + H 495.21889 121.0648 317.1536 13.91 Epitestosterone C19H28O2 M + H 289.21621 97.0648 109.0648 13.93 Estradiol benzoate C25H28O3 M + H 377.21112 279.1743 321.1849 12.22 Fludrocortisone acetate C23H31FO6 M + H 423.21774 181.1023 239.143 12.22 Flumetasone pivalate C27H36F2O6 M + H 495.25527 57.0699 121.0648 15.25 Flumethasone C22H28F2O5 M + H 411.19776 235.1117 277.1587 11.2 Fluocinolone acetonide C24H30F2O6 M + H 453.20832 121.0648 337.1434 11.86 Fluorometholone C22H29FO4 M + H 377.21226 279.1743 135.0804 12.22 Flurandrenolide C24H33FO6 M + H 437.23339 361.1798 105.0699 12.01 Fluticasone propionate C25H31F3O5S M + H 501.19171 121.0648 275.143 15.08 Nandrolone propionate C21H30O3 M + H 331.22677 257.18999 239.17943 17.38 Halcinonide C24H32ClFO5 M + H 455.19951 121.0648 377.1514 14.98 Dihydrodiethylstilbestrol C18H22O2 M + H 271.16926 109.1117 253.1587 11.92 Medroxyprogesterone C24H34O4 M + H 387.25299 123.0804 327.2319 15.81 acetate Megestrol acetate C24H32O4 M + H 385.23734 267.1743 224.156 15.53 Melengestrol acetate C25H32O4 M + H 397.23734 279.1743 337.2162 15.82 Methandienone C20H28O2 M + H 301.21621 283.20564 149.13248 12.73 Methylprednisolone C22H30O5 M + H 375.2166 161.0961 135.0804 10.99 Norethindrone C20H26O2 M + H 299.20056 109.0648 281.19 13.12 Nandrolone propionate C18H26O2 M + H 275.20056 109.06499 239.17885 12.47 Levonorgestrel C21H28O2 M + H 313.21621 109.0648 245.19 14.23 Prednicarbate C27H36O8 M + H 489.24829 147.0804 289.1587 14.91 Prednisolone C21H28O5 M + H 361.20095 147.0804 171.0804 10.2 Prednisone C21H26O5 M + H 359.1853 341.17474 237.12739 10.27 Progesterone C21H30O2 M + H 315.23186 109.06479 97.06479 15.76 Dehydrotestosterone C19H26O2 M + H 287.20056 121.0647 135.1167 12.22 Testosterone C19H28O2 M + H 289.21621 109.06479 97.06479 13.03 Trenbolone C18H22O2 M + H 271.16926 253.15869 199.11174 11.92 Triamcinolone C24H31FO6 M + H 435.21774 397.201 339.1591 11.69 acetonide Beclomethasone C22H29ClO5 M + H 409.17763 391.1671 279.1743 11.45 Betamethasone C28H37FO7 M + H 505.25961 279.1741 319.1693 15.45 dipropionate Betamethasone C27H37FO6 M + H 477.26469 279.1743 147.0804 14.55 valerate Betamethasone C22H29FO5 M + H 393.20718 355.19039 279.17434 11.14 Nitroimidazoles Dimetridazole C5H7N3O2 M + H 142.0611 112.06311 95.06037 7.17 Ipronidazole C7H11N3O2 M + H 170.0924 140.09441 123.09167 10.33 Metronidazole C6H9N3O3 M + H 172.07167 128.04545 82.05255 6.39 Ornidazole C7H10ClN3O3 M + H 220.04835 128.04547 203.14304 8.77 Hydroxydimetridazole C5H7N3O3 M + H 158.05602 140.0455 110.0475 6.42 Ronidazole C6H8N4O4 M + H 201.06183 140.04545 110.04746 7.03 Secnidazole C7H11N3O3 M + H 186.08732 128.0455 111.0427 7.56 Tinidazole C8H13N3O4S M + H 248.06995 154.0611 110.08384 8.12 Antiviral Amantadine C10H17N M + H 152.14338 135.11683 107.08553 7.48 drugs Rimantadine; C12H21N M + H 180.17468 163.14778 81.0704 9 Lincosamides Clindamycin C18H33ClN2O5S M + H 425.18715 126.12773 / 8.69 Lincomycin C18H34N2O6S M + H 407.221 126.12773 359.21766 6.76 Amphenicols Chloramphenicol C11H12C12N2O5 M − H 321.00505 257.03346 194.04588 10.11 Florfenicol C12H14Cl2FNO4S M − H 355.99319 335.98696 185.02779 9.96 Thiamphenicol C12H15Cl2NO5S M − H 353.99752 290.02593 185.02779 8.27 β-Lactams Ceftiofur C19H17N5O7S3 M + H 524.03629 241.03899 210.02065 9.63 Flucloxacillin C19H17ClFN3O5S M + H 454.06342 160.04242 114.03742 12.5 Cloxacillin C19H18ClN3O5S M + H 436.07285 277.0374 206.0367 12.26 Nafcillin C21H22N2O5S M + H 415.13222 199.0754 171.0441 12.54 Piperacillin C23H27N5O7S M + H 518.1704 143.0815 115.0502 10.16 Penicillin G C16H18N2O4S M + H 335.106 217.06414 176.07061 10.77 Quinoxalines Quinocetone C18H14N2O3 M + H 307.10772 143.06018 273.10178 11.99 Antipyretics Phenacetin C10H13NO2 M + H 180.10191 110.0602 138.09109 10.11 and analgesics Salicylic acid C7H6O3 M − H 137.02442 93.0346 65.0397 3.74 Amidopyrine C13H17N3O M + H 232.14444 113.10738 98.08418 6.46 Antipyrine C11H12N2O M + H 189.10224 147.09135 161.10696 8.39 Dapsone C12H12N2O2S M + H 249.06922 156.01122 108.04431 9.03 Flunixin meglumine C14H11F3N2O2 M + H 297.08454 279.07397 264.0505 13.66 Acetaminophen C8H9NO2 M + H 152.07061 110.06004 / 6.25 Psychoactive Haloperidol C21H23ClFNO2 M + H 376.14741 358.13685 165.07102 10.3 drugs Imipramine C19H24N2 M + H 281.20123 86.0963 236.1434 10.71 Nitrazepam C15H11N3O3 M + H 282.08732 268.08383 207.09126 11.53 Oxazepam C15H11ClN2O2 M + H 287.05818 241.05203 269.04703 11.49 Pemoline C9H8N2O2 M + H 177.06585 106.0651 79.0542 7.74 Perphenazine C21H26ClN3OS M + H 404.15579 143.11763 171.14877 10.83 Promethazine C17H20N2S M + H 285.142 198.0372 86.09643 10.34 Propionylpromazine C20H24N2OS M + H 341.16821 86.0964 58.0651 10.95 hydrochloride Sulpiride C15H23N3O4S M + H 342.1482 214.0169 112.1121 6.21 Xylazine C12H16N2S M + H 221.1107 90.0372 164.0528 8.27 hydrochloride Acepromazin C19H22N2OS M + H 327.15256 254.06341 86.09643 10.2 Anisopirol C19H24FN3O M + H 330.19762 312.1865 121.076 7.44 Azaperone C19H22FN3O M + H 328.18197 165.07069 121.07603 8.04 Carbamazepine C15H12N2O M + H 237.10224 194.0964 220.0757 11.15 Chloropromazine C17H19ClN2S M + H 319.10302 86.09643 246.01379 11.25 Diazepam C16H13ClN2O M + H 285.07892 154.0415 193.0883 13.58 Diphenhydramine C17H21NO M + H 256.16959 167.0855 152.0621 10.03 hydrochloride Droperidol C22H22FN3O2 M + H 380.17688 165.07072 194.09726 9.35 Estazolam C16H11ClN4 M + H 295.0745 267.05524 205.0755 11.44 Macrolides Clarithromycin C38H69NO13 M + H 748.48417 158.11756 116.07061 11.04 Tiamulin C28H47NO4S M + H 494.32986 192.10528 119.01613 10.8 Erythromycin C37H67NO13 M + H 734.46852 158.11756 116.07061 9.92 Kitasamycin C40H67NO14 M + H 786.46343 558.32727 174.11247 11.25 Tilmicosin C46H80N2O13 M + H 869.57332 174.11247 88.07569 8.93 Tylosin C46H77NO17 M + H 916.52643 772.44778 174.11247 10.17 anthelmintics Mebendazole C16H13N3O3 M + H 296.10297 264.07675 105.03349 10.85 Mebendazole-amine C14H11N3O M + H 238.09749 105.03369 / 8.45 2-aminofluorobenzimidazole C14H10FN3O M + H 256.08807 123.0241 113.0397 8.74 Oxfendazole C15H13N3O3S M + H 316.07504 284.04882 223.0576 9.26 Oxibendazole C12H15N3O3 M + H 250.11862 218.0924 176.04545 9.33 Parbendazole C13H17N3O2 M + H 248.13935 216.1131 160.0505 10.65 5-hydroxythiabendazole C10H7N3OS M + H 218.03826 191.02736 / 6.44 Tiabendazole C10H7N3S M + H 202.04334 175.03245 131.06037 7.12 Albendazole C12H15N3O2S M + H 266.09577 234.06956 191.01478 10.99 Albendazole sulfone C12H15N3O4S M + H 298.0856 266.05939 224.01244 9.4 Albendazole sulfoxide C12H15N3O3S M + H 282.09069 240.04347 208.01752 8.08 Amino albendazole C10H13N3O2S M + H 240.08012 198.03317 165.05328 6.94 sulfone Benzimidazole C7H6N2 M + H 119.06037 92.0495 65.0386 4.8 Febantel C20H22N4O6S M + H 447.13328 383.08085 280.05391 14.78 Lobendazole C10H11N3O2 M + H 206.0924 160.0505 178.0609 7.59 toltrazuril sulfoxide C18H14F3N3O5S M − H 440.05335 371.05813 / 8.97 Clopidol C7H7Cl2NO M + H 191.99775 86.9996 101.01525 6.83 Dinitolmide C8H7N3O5 M − H 224.03129 181.02438 77.03858 9.91 Halofuginone C16H17BrClN3O3 M + H 414.02146 100.07598 120.08076 8.89 Levamisole C11H12N2S M + H 205.0794 178.0685 / 6.99 Pesticides Buprofezin C16H23N3OS M + H 306.16346 201.10561 116.05285 17.73 Carboxin C12H13NO2S M + H 236.07398 143.01613 93.0573 12.61 3-hydroxycarbofuran C12H15NO4 M + H 238.10738 163.07536 135.08044 9.09 Clothianidin C6H8ClN5O2S M + H 250.016 131.96692 113.0168 8.96 Cyromazine C6H10N6 M + H 167.10397 85.05087 125.08217 3.63 Diuron C9H10Cl2N2O M + H 233.02429 72.04439 159.97153 12.65 Ethopabate C12H15NO4 M + H 238.10738 206.08117 164.07061 10.29 Fipronil C12H4Cl2F6N4OS M − H 434.93143 329.95845 249.95853 15.66 Fluridone C19H14F3NO M + H 330.11003 310.1038 290.09757 13.62 Acephate C4H10NO3PS M + H 184.01918 142.99264 112.99968 5.61 Hexazinone C12H20N4O2 M + H 253.1659 171.08765 71.06037 10.59 Imazalil C14H14C12N2O M + H 297.0556 158.97628 255.00864 10.77 Imidacloprid C9H10ClN5O2 M + H 256.05958 209.05885 175.09793 9.3 Linuron C9H10Cl2N2O2 M + H 249.01921 159.97153 182.02414 14.09 Metribuzin CSH14N4OS M + H 215.09611 187.10119 131.03859 11.73 Myclobutanil C15H17ClN4 M + H 289.12145 70.03997 125.01525 14.21 Acetamiprid C10H11ClN4 M + H 223.0745 126.0105 98.9997 9.58 Norflurazon C12H9ClF3N3O M + H 304.0459 284.03967 160.03686 12.81 Propyzamide C12H11Cl2NO M + H 256.02905 189.9821 172.95555 14.68 Atrazine C8H14ClN5 M + H 216.10105 174.0541 96.05562 12.63 Simazine C7H12ClN5 M + H 202.0854 132.0323 104.001 11.35 Desethyl atrazine C6H10ClN5 M + H 188.06975 146.0228 104.001 9.37 Azoxystrobin C22H17N3O5 M + H 404.1241 344.10397 329.07949 14.2 Benoxacor C11H11Cl2NO2 M + H 260.02396 149.08352 120.04439 14.31 Other Scopolamine C17H21NO4 M + H 304.15433 156.10191 138.09134 7 substances Valnemulin C31H52N2O5S M + H 565.36697 263.1417 72.08078 11.15 Atropine C17H23NO3 M + H 290.17507 124.11208 93.06988 7.61 Gradient elution program in positive ion mode Time (min) A(%) B(%) 0 95 5 1.0 95 5 15 5 95 17 5 95 17.1 95 5 22.0 95 5 Gradient elution program in negative ion mode Time (min) A(%) B(%) 0 95 5 8 5 95 10 5 95 10.1 95 5 13 95 5