METHOD OF MAKING MICROALGAL-BASED ANIMAL FOODSTUFF SUPPLEMENTS, MICROALGAL-SUPPLEMENTED ANIMAL FOODSTUFFS AND METHOD OF ANIMAL NUTRITION
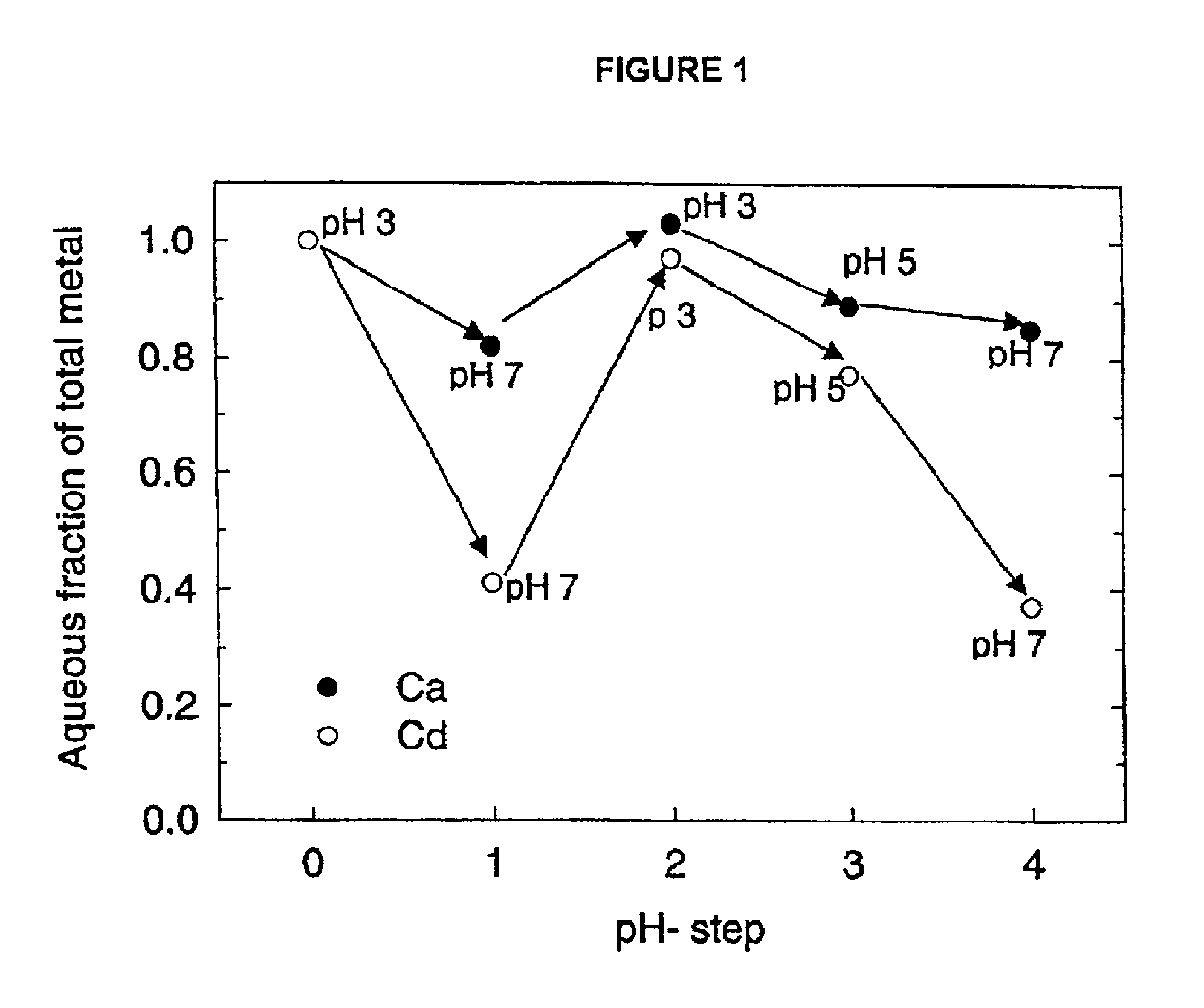
This application is a divisional application of U.S. application Ser. No. 09/765,142 filed Jan. 18, 2001, now U.S. Pat. No. ______, the contents of which is incorporated herein by reference. The present invention in the field of animal foodstuffs for the delivery of essential metals. The objective of the present invention is to produce a safe, biodegradable trace metal binding system that effectively delivers metals, such as chromium, copper, manganese, selenium and zinc to animals, including livestock such as cattle. The present invention generally involves the use of transgenic algae to express metal-specific binding peptides that release bound metals, typically at low pH. As used herein, release at low pH will be understood as meaning release at a pH that occurs somewhere within the digestive tract of the animal fed the foodstuff of the present invention, that pH being lower than that experienced by the foodstuff prior to ingestion by that animal. The present invention includes an animal foodstuff, a method of producing an animal foodstuff, and a method of providing an animal with nutrition. In general terms, the invention includes a method of preparing an animal foodstuff composition, the method comprising the steps: (a) providing transgenic algal cells comprising a nucleotide sequence, the nucleotide sequence capable of expressing a non-native metal-binding protein in the transgenic algal cells; (b) binding the metal-binding protein, with at least one metal so as to produce a metal-bound adduct of the metal-binding protein; and (c) admixing the metal-bound adduct with the animal foodstuff. The transgenic algal cells may be from any strain that may be genetically altered to carry the nucleotide sequence capable of expressing a non-native metal-binding protein. Examples include those selected from the genus The metal-binding protein may be adapted to bind any desired metal or combination of metals, such as those selected from the group consisting of cobalt, chromium, copper, iron, manganese, molybdenum, selenium and zinc, or combinations thereof. An example of a metal-binding protein is chicken Type II Metallothionein. It is preferred that the transgenic algal cells are in a dried state prior to introduction into the animal foodstuff. The present invention also includes an animal foodstuff composition comprising: (a) an animal foodstuff; and (b) transgenic algal cells, as described above, expressing a metal-binding protein not found in native algal cells, such that the transgenic algal cells contain the metal-binding protein, the metal-binding protein being bound to a metal. The foodstuff may be any natural or synthetic foodstuff or feedstock, such as grain or fodder for livestock, commercial pet foods for pet animals, etc. The present invention also includes a method of providing a dietary metal supplement to an animal, the method comprising feeding to the animal a foodstuff comprising transgenic algal cells expressing a metal-binding protein not found in native algal cells, as described above, such that the transgenic algal cells contain the metal-binding protein, the metal-binding protein being bound to a metal. With specific regard to copper or and zinc binding, one may determine the copper and zinc binding capacity of transgenic algae expressing metallothioneins. Presently, transgenic algae that express a chicken metallothionein-II gene fused to a plasma membrane protein have been generated. Results indicate that the metallothionein domain is expressed on the surface of the cell and effectively binds metals. Metallothioneins are cysteine-rich proteins that selectively sequester copper, zinc, lead, cadmium, cobalt, nickel, mercury, silver, and gold. Other metals such as calcium and iron do not bind to metallothioneins. Metallothioneins may be sub-divided into three classes based on their primary structures. Class I and II metallothioneins are low molecular weight (10 kD) translationally synthesized proteins, which have multiple CysXCys, or CysCys clusters. Each metallothionein binds 6-7 metal ions via formation of metal-thiolate clusters. The class III metallothioneins or phytochelatins are typically found in plants, algae, and fungi. Phytochelatins are non-translationally synthesized metal-thiolate polypeptides related to glutathione and have the repetitive sequence (γGluCys)nGly, where n=2-11. The trace metal binding constants are highest for the translationally synthesized metallothioneins (I and II) (K3=1025.5M−1) and are lower for the phytochelatins (K3=1019M−1). Significantly, metallothioneins bind metals with greatest affinity at neutral pH and release metals at low pH (Adhiya et al., Metallothionein is a general name given to heavy metal (Cd, Zn, Cu)-binding proteins having similar properties. They typically have low molecular weights, high contents of cysteinyl residues, and bind heavy metals tightly through mercaptide bonds. Metallothioneins (MTs) were discovered in 1957 when Margoshes and Vallee searched for a tissue component responsible for the natural accumulation of cadmium in mammalian kidney (Margoshes and Vallee 1957). Metallothioneins may be one of the few biological compounds known to specifically bind this metal. However, as documented already in the earliest reports, cadmium is but one of the several metallic components bound by MTs, the others being most commonly zinc and copper. Since the discovery of MTs, intense interest in this small, structurally unique, and functionally enigmatic group of proteins has been driven by its putative roles in cadmium detoxification and essential metal metabolism. The first MTs characterized were lower molecular eight, sulfur containing proteins, which consist of a single polypeptide chain of 61 amino acids residues. Twenty of the residues are cysteines, which chelate up to seven bivalent cations, leaving neither free thiol groups nor a disulphide bridge (Kojima, et al., 1976). Typically MTs also lack aromatic amino acids residues. All cysteines in MTs occur in the reduced form and are coordinated to the metal ions through mercaptide bonds, giving rise to spectroscopic features characteristic of metal-thiolate clusters (Martin, et al., 1992). In view of these unique chemical characteristics, the phenomenological definition was adopted that any polypeptide resembling mammalian MT in several of these features can be named an MT (Fowler et al., 1987). MTs now have been identified not only in the animal kingdom but also in higher plants, eukaryotic microorganisms, and in some prokaryotes (cited in Hamer 1986, Kagi and Kojima, 1987). To date, all organisms that have been screened contain one or more types of cysteine-rich polypeptides which selectively sequester heavy or soft metals including copper, zinc, lead, cadmium, cobalt, nickel, mercury, silver and gold (Grill et al., 1985; Jackson et al., 1987; Kagi and Schaffer, 1988; Rauser, 1990). Taking into account structural relationships, they can be subdivided into three classes (Fowler et al., 1987, Furey et al., 1986 Jackson et al., 1987; Rauser, 1990). Class I MTs (MT-I) include mammalian MTs and polypeptides with related primary structure from other phyla. They are characterized by (1) molecular weight of about 10 kD (equivalent to about 60 amino residues), (2) the presence of 20 invariant cysteine (Cys) residues arranged in CysXCys clusters, where X is any amino acid, (3) the absence of aromatic amino acid residues, and (4) the presence of 6-7 metal-thiolate clusters whose number varies with respect to the type of metal bound. Class II MTs (MT-II) are low molecular weight and Cys-rich metal binding proteins, but the distribution of Cys residues does not correspond to that in mammalian MTs. These proteins have been identified in sea urchin, wheat, yeast, and certain prokaryotes (Kagi, 1993). The third class of MTs (MT-III) are nontranslationally synthesized metallothiolate polypeptides related to glutathione and have repetitive sequences: (□-GluCys)nGly, where n=2-11 (Rauser, 1990). The MT-IIIs are also known as phytochelatins (PC), and have smaller molecular weights (1-2 kD) than MT-I or MT-IIs. Phytochelatins, as their name implies, are found in plants. Owing to improvements in protein sequencing techniques and the facile determination of nucleotide sequences, primary structure data are now available I some class I MTs, class II MTs and various homologous sets of class III MTs (Rauser, 1990; Kagi, et al., 1988). Table 1 shows some sequences of the three classes of MTs (Kagi, et al., 1988). All class I and class II MTs characterized thus far are single-chain proteins. Mammalian forms contain 61-62 amino acid residues; chicken MT and sea urchin MTs contain 63-64 residues, respectively. Shorter single chain proteins are found in invertebrates and in certain fungi, the shortest one with 25 residues in g-Glutamylcysteinylglycones detected in yeast, algae and certain plants are classified as class III MTs, or phytochelatins. The class III MTs are quite different in origin and structure although they show many phenomenological and functional similarities. Like the MTs of the higher organisms they are isolated from the cell cytosol as low-molecular weight, metal rich complexes. They are synthesized by enzymatic reactions rather than on ribosomes. Their structures involve unusual repeating sequences of γ-Glu linkages to cysteine, which produce the general formula: (γ-Glu-Cys)nGly ( The three classes of MTs are different in their affinities for heavy metals. The binding constants for cadmium range from K3=1025.5M−1for equine MT-I to K3=1019M−1for tobacco MT-II (Rauser, 1990). Another measure of metal binding affinity is the pH at which 50% of the bound metal is released from the protein. The higher the affinity for the metal, the lower the pH that is necessary to displace the metal from the protein. Typically, metal ions are displaced from MTs at pH values ranging from 2.0-4.5, with copper and cadmium generally being released at lower pH values. The Cd-MT from cabbage lost half its Cd at pH 4.4 (Wagner, 1984), the complex from tobacco did so at pH 5-5.8, whereas Cd-MT from rat liver dissociated by 50% at pH 3 (Reese, 1987). Kagi and Vallee (1988) found pH values of 3 and 4.5 for Cd and Zn dissociation, respectively, for equine Cd, Zn-MT. The pH-dependent selective release of heavy metals can also be used to selectively harvest heavy metals that vary with respect to their binding constants (Kagi and Schaffer, 1988; Rauser, 1990). Metallothioneins are an unusual group of proteins or polypeptides, which have challenged the interest of chemists and life scientists alike for over 30 years. In spite of the rich information on its structure, MT is a protein in search of functions (Karin 1985). More than three decades after the discovery of MT, its functional significance remains a topic of discussion (Karin, 1985: Bremner, 1987). Questions remain with respect to how cadmium exerts its toxicity and its mechanism for exercising its defensive action. The conservation of the structure, the ubiquitous occurrence, and the programmed synthesis of MT in regeneration and development are strong arguments for its playing a crucial role in some fundamental metal-related cell biological process. The main hypotheses thus far considered are that: (1) MT serves as a relatively non-specified metal-buffering ligand to either sequester or dispense metal ions, or (2) it has a specialized function in normal cellular metabolism or development (Kagi and Schaffer, 1988). It may also serve a number of different biological purposes. That MT is the cellular component responsible for much of the intracellular sequestration of Cd, bringing about the long biological half-life of this nonessential element, is unquestioned (Webb, 1987a). Through gene amplification (Griffith et al., 1983, Durnam and Palmiter 1987) and transfer (Thiele et al. 1986, Ecker et al. 1986) experiments, the presence of MT is shown to be strongly associated with Cd detoxification. In cultured mammalian cell lines stable resistance to Cd can be brought about by massive amplification of the MT genes (Beach, et al., 1981). However, the production of excessive amounts of Cd-containing MT has been suggested as a causative factor in bringing about kidney damage in chronic Cd poisoning (Nordberg, et al., 1987), thus causing doubt on the biological importance of MT synthesis as a specific and effective Cd defense mechanism in animals. MTs have also been implicated in the sequestration of other nonessential metals, such as Hg, Ag, Au, Pt and Pb (Webb, et al., 1987a). Such effects have been claimed to be responsible for the development of resistance toward Au- and Pt-containing drugs in cultured cells and the selective protection of some tissue from such agents in animals following preinduction of MT (Naganuma et al., 1987; Monia et al., 1987). Hypersensitivity to elevated trace-metal concentrations has also been observed in fungal (Hamer, 1986) and prokaryotic (Turner, et. al., 1993) cells with deleted MT genes, selected for enhanced tolerance to certain trace metal ions. The preponderance of Zn in most MT preparations and the responsiveness of MT-bound Zn and Cu to the dietary supplies of these essential nutrients suggest a role in their metabolism and have led to a large number of studies (cited in Bremner 1987). As a homeostatic mediator, MT could donate metal ions in the biosynthesis of Zn and Cu-containing metalloenzymes and metalloproteins (Brady, 1982). The emergence of Cu-MT in A biological role, probably unrelated to a detoxification function for metals, is suggested by the fact that in certain tissues and cell types MT is induced by chemical and physical stress. These effects, which are most prominent in liver and mediated in part by hormones, resemble an acute phase response (Bremner, 1987). In some cases, such as in the exposure to electrophilic agents, i.e., O2, free radicals, and alkylating agents, the increased supply of MT could provide neutralizing nucleophilic equivalents. However, in most other instances it is unclear how the organism benefits from increased MT biosynthesis. One possibility is that MTs may have metalloregulation function in cellular repair processes, growth, and differentiation. This was first suggested by the parallelism of enhanced RNA synthesis with increased Zn-MT formation observed in the liver of rats recovering from partial hepatectomy (Ohtake et al., 1978) and is supported by the programmed regulation of MT mRNA and protein levels during embryogenesis (Nemer et al., 1984) and at different stages of fetal and prenatal development (Andrews, et al., 1984). In view of the known effects of Zn on embryogenesis, its participation in RNA polymerases and its serving as a structural modulator of the Zn finger domain in several DNA-binding proteins, it is tempting to hypothesize that Zn-MT plays a part in expression of genetic information. While all plants examined to data contain phytochelatins, apparently, only a few contain class I and class II MTs. An MT-I has been identified in immature embryos of wheat and MT-I has been identified in barley, pea, In accordance with the foregoing summary, the following presents a detailed description of the preferred embodiment of the invention that is currently considered to be the best mode. The copper and zinc binding capacity of the transgenic algae expressing metallothionein may be determined and quantified prior to the production of the animal foodstuff of the present invention. Metal binding may be quantified by ICP-mass spectrometry and replicate experiments may be carried out to determine statistical significance. Titration of pH-dependent metal binding using both live and dead cells may also be done in order to determine the preferred candidate(s), depending upon the desired application. An example of the pH-dependent binding of cadmium treated cells is shown in Studies indicate that there are cadmium-specific binding sites on the cell surfaces of Unique metal-binding peptides (MBP) that sequester specific trace metals (chromium, copper, manganese, selenium and zinc) with high affinity and in a pH dependent manner may be identified. These MBPs may be expressed on the cell surface of The Identification of Oligopeptides that Selectively Bind Trace Metals A variety of strategies have been developed to identify MBPs. Metal-binding domains have been identified in naturally occurring metallo-proteins (such as metallothioneins and trace metal chaperonins) by structural and functional analysis using chemical modification, site-directed mutagenesis, NMR, and crystallographic approaches (Brown, S., A more rapid approach for identification of MBPs involves screening combinatorial DNA or oligopeptide libraries in phages which display potential MBPs on the surface of the coat protein (Kotrba, P. et al., Using the New England Biolabs Ph.D. 7 (heptamer) combinatorial phage library MBPs using copper- and lead-IDA resins have been screened for. Copper and lead specific MPB-phages were eluted by a pH shift from 7.0 to 4.0. Ten MBP-phages that bind to lead-IDA columns and ten MBP-phages that bind to a copper-IDA column have been purified. The determination of the DNA sequence of the region encoding the heptamers may be made. The translated protein sequences then may be compared to each other and protein data banks to determine if there is a consensus domain. Previously identified MBP sequences as well as biopan for chromium, copper, manganese, selenium and zinc binding phages using techniques currently in use may be exploited. Many novel MBPs as well as MBPs that are most compatible as protein fusions with Characterization of Trace Metal Binding Properties of Oligopeptides and their Tandem Repeats Following identification of the MBPs determination as to the metal binding constants (affinities) of the MBP-phages by equilibrium dialysis may be made (Adhiya et al., Some possible gene (protein) fusion constructs would include the relevant MBPs listed in the table fused to the N-terminal and C-terminal domain regions of the All gene fusion constructs may be introduced into Following verification of the expression of the MBP-fusion proteins, The possibility of introducing multiple metal-binding domains into a single gene fusion as tandem repeats (separated by spacer regions (Gly-Gly)) may be explored. It has been observed that tandem repeat MBP constructs may not enhance metal binding, however (Kotrba, P. et al., Since the MBDs are small these constructs should not interfere with proper targeting and folding of Gene constructs encoding trace metal binding factors also may be integrated into the genomes of wild type as well as transgenic Once the transgenic algae have been identified that bind each of the target metals in a pH-dependent manner they may be provided for field testing studies in cattle. The preferred embodiments herein disclosed are not intended to be exhaustive or to unnecessarily limit the scope of the invention. The preferred embodiments were chosen and described in order to explain the principles of the present invention so that others skilled in the art may practice the invention. Having shown and described preferred embodiments of the present invention, it will be within the ability of one of ordinary skill in the art to make alterations or modifications to the present invention, such as through the substitution of equivalent materials or structural arrangements, or through the use of equivalent process steps, so as to be able to practice the present invention without departing from its spirit as reflected in the appended claims, the text and teaching of which are hereby incorporated by reference herein. It is the intention, therefore, to limit the invention only as indicated by the scope of the claims and equivalents thereof: The present invention is to a safe, biodegradable trace metal binding system that effectively delivers chromium, cobalt, copper, iron, manganese, molybdenum, selenium and zinc to animals. The method of preparing an animal foodstuff composition involves the steps of: providing transgenic algal cells comprising a nucleotide sequence, the nucleotide sequence being capable of expressing a non-native metal-binding protein in the transgenic algal cells; binding the metal-binding protein with at least one metal so as to produce a metal-bound adduct of the metal-binding protein; and admixing the metal-bound adduct with animal foodstuff. The invention is also to a animal foodstuff composition comprising animal foodstuff and transgenic algal cells expressing a non-native metal-binding protein in the transgenic algal cells, such that the transgenic algal cells contain the metal-binding protein and the metal-binding protein being bound to a metal. 1. A method of preparing an animal foodstuff composition, said method comprising the steps:
(a) providing algal cells comprising a nucleotide sequence, said nucleotide sequence capable of expressing a non-native metal-binding protein in said algal cells; (b) binding said metal-binding protein with at least one metal so as to produce a metal-bound adduct of said metal-binding protein; and (c) admixing said metal-bound adduct with said animal foodstuff. 2. A method according to 3. A method according to 4. A method according to 5. An animal foodstuff composition comprising:
(a) an animal foodstuff; and (b) algal cells expressing a non-native metal-binding protein in said algal cells, such that said algal cells contain said metal-binding protein, said metal-binding protein being bound to a metal. 6. An animal foodstuff composition of 7. An animal foodstuff composition according to 8. An animal foodstuff composition according to 9. A method of providing a dietary metal supplement to an animal, said method comprising feeding to said animal a food stuff comprising algal cells expressing a non-native metal-binding protein, such that said algal cells contain said metal-binding protein, said metal-binding protein being bound to a metal.RELATED APPLICATIONS
TECHNICAL FIELD OF THE INVENTION
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
Metallothionein
DEFINITION AND OCCURRENCE
Primary Structure of MTs
Class I Human MDP-NCSCAAGDSCTCAGS CKCKECTKCTS CKKSCC SCCBVG-CAKCAQGCI-CKJACDKCSCCA Chicken MDPQDCTCAAGGSCSCAGS CKCKMCR-CRRSCRKSCC SCCPAG-CNNCAKGCVCKEPASSKCSCCH Trout MDP--CECSKTGS CNCGGSCKCSN CA-CTSCKKS C CPCCPSD-CK-CASGCVCKGKTC DTSCCQ Crab PDP---C-C--NDKCDSKEGECKTG-CK-CTSCRCPPC EQCSSG--CK-CANKEGCRKTCSKPCSCCP --GDCGC SGASS--CNCGSG-CS---CSMCGSK Class II Sea urchin MPDVKCVCCTEGKECACFGQDCCVTGECCKDGTCCGI CINAACKCANGCKCGSGCSCTEGNCA Yeast QNEGHECQCQCGSCKMNEQCKKSCSCPTGCNSDDKCP CGMKCEETKKSCCSGK Wheat GCNDKCGCAVPCPGGTGCRCTSARCGAAAGEHTTCGC GEHCGGNPCACGGGEGTPSGCAN Synechocystis TSTTLVKCACEPCLCNVDPSKAIDRNGLYY CCEACADGHTGGKGCGHTGCNC Class III ECECECG ECECECECECECECECG ECECECECECECEC-b-alanine Biological Functions
metal ions: Cd, Zn, Cu, Hg, Au, Streptozotocin Ag, Co, Ni, Bi. 2-propanol glucocorticoids ethanol progesterone ethionine nitrogen alkylating agents glucagon chloroform catecholamines starvation interleukin I infection interferon physical stress endotoxin X-irradiation dextran high O2tesion for citations, see Palmiter (1987) and Bremner (1987). Molecular Biology of MTs
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT(S)
Nickel HHHHHH, WHHHPH, AQHHHH, CAIH, GGH Copper HHHHHH, SPHHGG, HHHMVH, AMLKLH Chromium QHQK Gold MHGKTQATSGTIQS Expression of Trace Metal Binding Oligopeptides on